Postnatal NG2 proteoglycan-expressing progenitor cells are intrinsically multipotent and generate functional neurons
- PMID: 12682089
- PMCID: PMC2172886
- DOI: 10.1083/jcb.200210110
Postnatal NG2 proteoglycan-expressing progenitor cells are intrinsically multipotent and generate functional neurons
Abstract
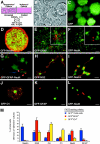
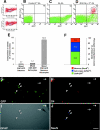
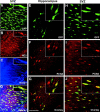
Neurogenesis is known to persist in the adult mammalian central nervous system (CNS). The identity of the cells that generate new neurons in the postnatal CNS has become a crucial but elusive issue. Using a transgenic mouse, we show that NG2 proteoglycan-positive progenitor cells that express the 2',3'-cyclic nucleotide 3'-phosphodiesterase gene display a multipotent phenotype in vitro and generate electrically excitable neurons, as well as astrocytes and oligodendrocytes. The fast kinetics and the high rate of multipotent fate of these NG2+ progenitors in vitro reflect an intrinsic property, rather than reprogramming. We demonstrate in the hippocampus in vivo that a sizeable fraction of postnatal NG2+ progenitor cells are proliferative precursors whose progeny appears to differentiate into GABAergic neurons capable of propagating action potentials and displaying functional synaptic inputs. These data show that at least a subpopulation of postnatal NG2-expressing cells are CNS multipotent precursors that may underlie adult hippocampal neurogenesis.
Figures







References
-
- Altman, J., and G.D. Das. 1965. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J. Comp. Neurol. 124:319–335. - PubMed
-
- Alvarez-Buylla, A., and S. Temple. 1998. Stem cells in the developing and adult nervous system. J. Neurobiol. 36:105–110. - PubMed
-
- Alvarez-Buylla, A., J.M. Garcia-Verdugo, and A.D. Tramontin. 2001. A unified hypothesis on the lineage of neural stem cells. Nat. Rev. Neurosci. 2:287–293. - PubMed
-
- Bansal, R., and S.E. Pfeiffer. 1997. a. FGF-2 converts mature oligodendrocytes to a novel phenotype. J. Neurosci. Res. 50:215–228. - PubMed
-
- Bansal, R., and S.E. Pfeiffer. 1997. b. Regulation of oligodendrocyte differentiation by fibroblast growth factors. Adv. Exp. Med. Biol. 429:69–77. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

