DC-SIGN (CD209) mediates dengue virus infection of human dendritic cells
- PMID: 12682107
- PMCID: PMC2193896
- DOI: 10.1084/jem.20021840
DC-SIGN (CD209) mediates dengue virus infection of human dendritic cells
Abstract
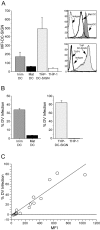
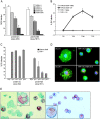
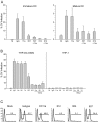
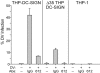
Dengue virus is a single-stranded, enveloped RNA virus that productively infects human dendritic cells (DCs) primarily at the immature stage of their differentiation. We now find that all four serotypes of dengue use DC-SIGN (CD209), a C-type lectin, to infect dendritic cells. THP-1 cells become susceptible to dengue infection after transfection of DC-specific ICAM-3 grabbing nonintegrin (DC-SIGN), or its homologue L-SIGN, whereas the infection of dendritic cells is blocked by anti-DC-SIGN antibodies and not by antibodies to other molecules on these cells. Viruses produced by dendritic cells are infectious for DC-SIGN- and L-SIGN-bearing THP-1 cells and other permissive cell lines. Therefore, DC-SIGN may be considered as a new target for designing therapies that block dengue infection.
Figures
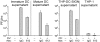
References
-
- Monath, T.P., and F.X. Heinz. 1996. Flaviviruses. In Fields Virology. B.N. Fields, D.M. Knipe, and P.M. Howley, editors. Lippincott-Raven, Philadelphia. 961–1034.
-
- Gubler, D.J., S. Nalim, R. Tan, H. Saipan, and J. Sulianti Saroso. 1979. Variation in susceptibility to oral infection with dengue viruses among geographic strains of Aedes aegypti. Am. J. Trop. Med. Hyg. 28:1045–1052. - PubMed
-
- Halstead, S.B. 1997. Epidemiology of dengue and dengue hemorrhagic fever. In Dengue and Dengue Hemorrhagic Fever. D.J. Gubler and G. Kuno, editors. Cab International, London. 23–44.
-
- Burke, D.S., A. Nisalak, D.E. Johnson, and R.M. Scott. 1988. A prospective study of dengue infections in Bangkok. Am. J. Trop. Med. Hyg. 38:172–180. - PubMed
-
- Kliks, S.C., A. Nisalak, W.E. Brandt, L. Wahl, and D.S. Burke. 1989. Antibody-dependent enhancement of dengue virus growth in human monocytes as a risk factor for dengue hemorrhagic fever. Am. J. Trop. Med. Hyg. 40:444–451. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

