CD1d-expressing dendritic cells but not thymic epithelial cells can mediate negative selection of NKT cells
- PMID: 12682110
- PMCID: PMC2193895
- DOI: 10.1084/jem.20021366
CD1d-expressing dendritic cells but not thymic epithelial cells can mediate negative selection of NKT cells
Abstract
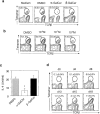
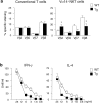
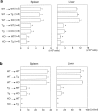
Natural killer T (NKT) cells are a unique immunoregulatory T cell population that is positively selected by CD1d-expressing thymocytes. Previous studies have shown that NKT cells exhibit autoreactivity, which raises the question of whether they are subject to negative selection. Here, we report that the addition of agonist glycolipid alpha-galactosylceramide (alpha-GalCer) to a fetal thymic organ culture (FTOC) induces a dose-dependent disappearance of NKT cells, suggesting that NKT cells are susceptible to negative selection. Overexpression of CD1d in transgenic (Tg) mice results in reduced numbers of NKT cells, and the residual NKT cells in CD1d-Tg mice exhibit both an altered Vbeta usage and a reduced sensitivity to antigen. Furthermore, bone marrow (BM) chimeras between Tg and WT mice reveal that CD1d-expressing BM-derived dendritic cells, but not thymic epithelial cells, mediate the efficient negative selection of NKT cells. Thus, our data suggest that NKT cells developmentally undergo negative selection when engaged by high-avidity antigen or abundant self-antigen.
Figures







Similar articles
-
The interface between innate and acquired immunity: glycolipid antigen presentation by CD1d-expressing dendritic cells to NKT cells induces the differentiation of antigen-specific cytotoxic T lymphocytes.Int Immunol. 2000 Jul;12(7):987-94. doi: 10.1093/intimm/12.7.987. Int Immunol. 2000. PMID: 10882410
-
Human invariant V alpha 24-J alpha Q TCR supports the development of CD1d-dependent NK1.1+ and NK1.1- T cells in transgenic mice.J Immunol. 2003 Mar 1;170(5):2390-8. doi: 10.4049/jimmunol.170.5.2390. J Immunol. 2003. PMID: 12594262
-
A subset of NKT cells that lacks the NK1.1 marker, expresses CD1d molecules, and autopresents the alpha-galactosylceramide antigen.J Immunol. 2000 Nov 1;165(9):4917-26. doi: 10.4049/jimmunol.165.9.4917. J Immunol. 2000. PMID: 11046017
-
Regulation of immune responses by CD1d-restricted natural killer T cells.Immunol Res. 2004;30(2):139-53. doi: 10.1385/IR:30:2:139. Immunol Res. 2004. PMID: 15477656 Review.
-
The Role of Adaptor Proteins in the Biology of Natural Killer T (NKT) Cells.Front Immunol. 2019 Jun 25;10:1449. doi: 10.3389/fimmu.2019.01449. eCollection 2019. Front Immunol. 2019. PMID: 31293596 Free PMC article. Review.
Cited by
-
Type II NKT cells: a distinct CD1d-restricted immune regulatory NKT cell subset.Immunogenetics. 2016 Aug;68(8):665-76. doi: 10.1007/s00251-016-0930-1. Epub 2016 Jul 12. Immunogenetics. 2016. PMID: 27405300 Free PMC article. Review.
-
Co-receptor choice by V alpha14i NKT cells is driven by Th-POK expression rather than avoidance of CD8-mediated negative selection.J Exp Med. 2010 May 10;207(5):1015-29. doi: 10.1084/jem.20090557. Epub 2010 Apr 19. J Exp Med. 2010. PMID: 20404101 Free PMC article.
-
Development of mucosal-associated invariant T cells.Immunol Cell Biol. 2018 Jul;96(6):598-606. doi: 10.1111/imcb.12039. Epub 2018 Apr 24. Immunol Cell Biol. 2018. PMID: 29569752 Free PMC article. Review.
-
The adaptor protein SAP regulates type II NKT-cell development, cytokine production, and cytotoxicity against lymphoma.Eur J Immunol. 2014 Dec;44(12):3646-57. doi: 10.1002/eji.201444848. Epub 2014 Oct 28. Eur J Immunol. 2014. PMID: 25236978 Free PMC article.
-
Nur77 controls tolerance induction, terminal differentiation, and effector functions in semi-invariant natural killer T cells.Proc Natl Acad Sci U S A. 2020 Jul 21;117(29):17156-17165. doi: 10.1073/pnas.2001665117. Epub 2020 Jul 1. Proc Natl Acad Sci U S A. 2020. PMID: 32611812 Free PMC article.
References
-
- Bendelac, A., M.N. Rivera, S.H. Park, and J.H. Roark. 1997. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu. Rev. Immunol. 15:535–562. - PubMed
-
- Eberl, G., R. Lees, S.T. Smiley, M. Taniguchi, M.J. Grusby, and H.R. MacDonald. 1999. Tissue-specific segregation of CD1d-dependent and CD1d-independent NK T cells. J. Immunol. 162:6410–6419. - PubMed
-
- Hammond, K.J., S.B. Pelikan, N.Y. Crowe, E. Randle-Barrett, T. Nakayama, M. Taniguchi, M.J. Smyth, I.R. van Driel, R. Scollay, A.G. Baxter, and D.I. Godfrey. 1999. NKT cells are phenotypically and functionally diverse. Eur. J. Immunol. 29:3768–3781. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

