DNA fingerprinting of Salmonella enterica subsp. enterica serovar typhimurium with emphasis on phage type DT104 based on variable number of tandem repeat loci
- PMID: 12682132
- PMCID: PMC153889
- DOI: 10.1128/JCM.41.4.1469-1479.2003
DNA fingerprinting of Salmonella enterica subsp. enterica serovar typhimurium with emphasis on phage type DT104 based on variable number of tandem repeat loci
Abstract
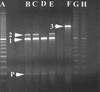
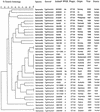
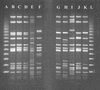
Seventy-eight human and environmental strains of Salmonella enterica subsp. enterica serovar Typhimurium, as well as 18 isolates of other Salmonella serovars and 6 isolates of Escherichia coli, were subjected to a novel variable number of tandem repeats (VNTR)-based fingerprinting method that showed high discrimination and reproducibility for typing serovar Typhimurium isolates. The method is based on capillary separation of PCR products from fluorescence-labeled VNTR in the serovar Typhimurium genome. The serovar Typhimurium isolates displayed 54 VNTR patterns, and the VNTR assay correctly identified strains from a well-characterized outbreak. Among 37 serovar Typhimurium phage type DT104 isolates, 28 distinct VNTR patterns were found. This VNTR-based method is fast and suitable for complete automation. Our VNTR-based method was capable of high discrimination within the homogeneous serovar Typhimurium DT104 phage type and can be used to trace outbreaks and to monitor DT104 as well as other phage types. The VNTR assay was compared to XbaI pulsed-field gel electrophoresis, amplified fragment length polymorphism analysis, integron-cassette profiles and gene PCR of intI1, qacEDelta1, sulI1, and floR. The VNTR assay showed greatly improved resolution compared to all other tested methods in this study.
Figures




Similar articles
-
Multilocus variable number tandem repeat analysis for Salmonella enterica subspecies.Eur J Clin Microbiol Infect Dis. 2011 Apr;30(4):465-73. doi: 10.1007/s10096-010-1110-0. Epub 2010 Dec 11. Eur J Clin Microbiol Infect Dis. 2011. PMID: 21153561 Review.
-
Characterization of Salmonella enterica serovar Typhimurium and monophasic Salmonella serovar 1,4,[5],12:i:- isolates in Thailand.J Clin Microbiol. 2005 Jun;43(6):2736-40. doi: 10.1128/JCM.43.6.2736-2740.2005. J Clin Microbiol. 2005. PMID: 15956391 Free PMC article.
-
Variable number of tandem repeats in Salmonella enterica subsp. enterica for typing purposes.J Clin Microbiol. 2004 Dec;42(12):5722-30. doi: 10.1128/JCM.42.12.5722-5730.2004. J Clin Microbiol. 2004. PMID: 15583305 Free PMC article.
-
Molecular characterization of multidrug-resistant Salmonella enterica subsp. enterica serovar Typhimurium isolates from swine.J Clin Microbiol. 2002 Aug;40(8):2813-22. doi: 10.1128/JCM.40.8.2813-2822.2002. J Clin Microbiol. 2002. PMID: 12149335 Free PMC article.
-
Genome Variation and Molecular Epidemiology of Salmonella enterica Serovar Typhimurium Pathovariants.Infect Immun. 2018 Jul 23;86(8):e00079-18. doi: 10.1128/IAI.00079-18. Print 2018 Aug. Infect Immun. 2018. PMID: 29784861 Free PMC article. Review.
Cited by
-
Multilocus variable number tandem repeat analysis for Salmonella enterica subspecies.Eur J Clin Microbiol Infect Dis. 2011 Apr;30(4):465-73. doi: 10.1007/s10096-010-1110-0. Epub 2010 Dec 11. Eur J Clin Microbiol Infect Dis. 2011. PMID: 21153561 Review.
-
Evaluation and strategy for use of MIRU-VNTRplus, a multifunctional database for online analysis of genotyping data and phylogenetic identification of Mycobacterium tuberculosis complex isolates.J Clin Microbiol. 2008 Aug;46(8):2692-9. doi: 10.1128/JCM.00540-08. Epub 2008 Jun 11. J Clin Microbiol. 2008. PMID: 18550737 Free PMC article.
-
Tandem repeat analysis for surveillance of human Salmonella Typhimurium infections.Emerg Infect Dis. 2007 Mar;13(3):388-95. doi: 10.3201/eid1303.060460. Emerg Infect Dis. 2007. PMID: 17552091 Free PMC article.
-
Impact of compounding error on strategies for subtyping pathogenic bacteria.Foodborne Pathog Dis. 2008 Aug;5(4):505-16. doi: 10.1089/fpd.2008.0097. Foodborne Pathog Dis. 2008. PMID: 18713065 Free PMC article.
-
Multilocus variable-number tandem-repeat method for typing Salmonella enterica serovar Newport.J Clin Microbiol. 2009 Jun;47(6):1934-8. doi: 10.1128/JCM.00252-09. Epub 2009 Apr 22. J Clin Microbiol. 2009. PMID: 19386855 Free PMC article.
References
-
- Armour, J. A., and A. J. Jeffreys. 1992. Biology and applications of human minisatellite loci. Curr. Opin. Genet. Dev. 2:850-856. - PubMed
-
- Carlson, S. A., W. C. Stoffregen, and S. R. Bolin. 2002. Abomasitis associated with multiple antibiotic resistant Salmonella enterica serotype Typhimurium phagetype DT104. Vet. Microbiol. 85:233-240. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

