Clathrin-coated pits with long, dynamin-wrapped necks upon expression of a clathrin antisense RNA
- PMID: 12682302
- PMCID: PMC154318
- DOI: 10.1073/pnas.0534231100
Clathrin-coated pits with long, dynamin-wrapped necks upon expression of a clathrin antisense RNA
Abstract
To investigate the role of clathrin in coated vesicle formation, a cell line with inducible expression of clathrin heavy chain (CHC) antisense RNA was produced. After 18 h of CHC antisense RNA expression, the internalization of transferrin was inhibited by 90%. Although the amount of CHC was reduced by only 10%, the frequency of clathrin-coated pits at the cell surface increased by a factor of 3-5, and clathrin-coated structures also accumulated on a pleiomorphic, multivesicular, endosomal compartment. Remarkably, the coated pits were connected to the cell surface by long, tubular necks wrapped by dynamin rings, and the level of dynamin in the CHC antisense RNA-expressing cells was up-regulated 10-fold. In contrast, the amount of several other proteins associated with clathrin coat formation was unaffected. Thus, this study demonstrates that CHC antisense RNA causes accumulation of clathrin-coated pits with dynamin rings around the neck in intact cells not transfected with dynamin mutants, suggesting the existence of a previously uncharacterized functional interplay between clathrin and dynamin.
Figures

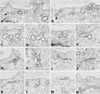
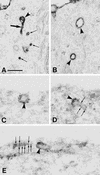
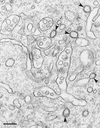

Comment in
-
Does clathrin pull the fission trigger?Proc Natl Acad Sci U S A. 2003 Apr 29;100(9):4981-3. doi: 10.1073/pnas.0930650100. Epub 2003 Apr 18. Proc Natl Acad Sci U S A. 2003. PMID: 12704235 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

