Tuning of conformational preorganization in model 2',5'- and 3',5'-linked oligonucleotides by 3'- and 2'-O-methoxyethyl modification
- PMID: 12682357
- PMCID: PMC153733
- DOI: 10.1093/nar/gkg305
Tuning of conformational preorganization in model 2',5'- and 3',5'-linked oligonucleotides by 3'- and 2'-O-methoxyethyl modification
Abstract
Conformational properties of trimeric and tetrameric 2',5'-linked oligonucleotides, 3'-MOE-A3(2',5') (1) and 3'-MOE-A4(2',5') (2), and their 3',5'-linked analogs, 2'-MOE-A3(3',5') (3) and 2'-MOE-A4(3',5') (4), were examined with the use of heteronuclear NMR spectroscopy. The temperature-dependent 3JHH, 3JHP and 3JCP coupling constants, acquired in the range of 273-343 K, gave insight into the conformation of sugar rings in terms of a two-state North <---> South (N <---> S) pseudorotational equilibrium and into the conformation of the sugar-phosphate backbone in the model antisense oligonucleotides 1-4. 2',5'-linked oligomers 3'-MOE-A3(2',5') (1) and 3'-MOE-A4(2',5') (2) show preference for N-type conformers and indication of A-type conformational features, which is prerequisite for antisense hybridization. The drive of N <---> S equilibrium in 1-4 has been rationalized with the competing gauche effects of 2'/3'-phosphodiester and 3'/2'-MOE groups, anomeric and steric effects. Furthermore, the pairwise comparisons of 3'-MOE with 3'-OH and 3'-deoxy 2',5'-linked adenine trimers emphasized the fine tuning of N <---> S equilibrium in 3'-MOE-A3(2',5') (1) and 3'-MOE-A4(2',5') (2) by the steric effects of 3'-MOE group and the possibility of water-mediated H-bonds with vicinal phosphodiester functionality. In full correspondence, the drive of N <---> S equilibrium towards N by 2'-MOE in 3',5'-linked analogs 2'-MOE-A3(3',5') (3) and 2'-MOE-A4(3',5') (4) is weaker in comparison with 3'-OH group in the corresponding ribo analogs. Beta(t), gamma+ and epsilon- rotamers are preferred in both 2',5'- and in 3',5'-linked oligonucleotides 1-4.
Figures
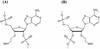
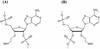



Similar articles
-
Solution structure of a modified 2',5'-linked RNA hairpin involved in an equilibrium with duplex.Nucleic Acids Res. 2005 Mar 23;33(6):1749-59. doi: 10.1093/nar/gki318. Print 2005. Nucleic Acids Res. 2005. PMID: 15788747 Free PMC article.
-
Double sugar and phosphate backbone-constrained nucleotides: synthesis, structure, stability, and their incorporation into oligodeoxynucleotides.J Org Chem. 2009 May 1;74(9):3248-65. doi: 10.1021/jo900391n. J Org Chem. 2009. PMID: 19348480
-
NMR structure of 2'-O-(2-methoxyethyl) modified and C5-methylated RNA dodecamer duplex.Biochimie. 2013 Dec;95(12):2385-91. doi: 10.1016/j.biochi.2013.08.025. Epub 2013 Sep 3. Biochimie. 2013. PMID: 24012551
-
The rotating frame nuclear Overhauser effect spectroscopy experiment as an aid to determining solution structures of DNA oligomers.Biopolymers. 2000 Jul;54(1):35-43. doi: 10.1002/(SICI)1097-0282(200007)54:1<35::AID-BIP40>3.0.CO;2-1. Biopolymers. 2000. PMID: 10799979 Review.
-
An overview of sugar-modified oligonucleotides for antisense therapeutics.Chem Biodivers. 2011 Sep;8(9):1616-41. doi: 10.1002/cbdv.201100081. Chem Biodivers. 2011. PMID: 21922654 Review.
Cited by
-
Solution-state structure of a fully alternately 2'-F/2'-OMe modified 42-nt dimeric siRNA construct.Nucleic Acids Res. 2010 Nov;38(20):7298-307. doi: 10.1093/nar/gkq621. Epub 2010 Jul 12. Nucleic Acids Res. 2010. PMID: 20624819 Free PMC article.
-
2-Thiouracil deprived of thiocarbonyl function preferentially base pairs with guanine rather than adenine in RNA and DNA duplexes.Nucleic Acids Res. 2015 Mar 11;43(5):2499-512. doi: 10.1093/nar/gkv109. Epub 2015 Feb 17. Nucleic Acids Res. 2015. PMID: 25690900 Free PMC article.
-
Solution structure of a modified 2',5'-linked RNA hairpin involved in an equilibrium with duplex.Nucleic Acids Res. 2005 Mar 23;33(6):1749-59. doi: 10.1093/nar/gki318. Print 2005. Nucleic Acids Res. 2005. PMID: 15788747 Free PMC article.
References
-
- Agrawal S. and Zhao,Q.Y. (1998) Antisense therapeutics. Curr. Opin. Chem. Biol., 2, 519–528. - PubMed
-
- Manoharan M. (1999) 2′-Carbohydrate modifications in antisense oligonucleotide therapy: importance of conformation, configuration and conjugation. Biochim. Biophys. Acta, 1489, 117–130. - PubMed
-
- Manoharan M., Kawasaki,A.M., Prakash,T.P., Fraser,A.S., Prhavc,M., Inamati,G.B., Casper,M.D. and Cook,P.D. (1999) Carbohydrate modifications in antisense oligonucleotide therapy: new kids on the block. Nucl. Nucl., 18, 1737–1746.
-
- Teplova M., Minasov,G., Tereshko,V., Inamati,G.B., Cook,P.D., Manoharan,M. and Egli,M. (1999) Crystal structure and improved antisense properties of 2′-O-(2-methoxyethyl)-RNA. Nature Struct. Biol., 6, 535–539. - PubMed
-
- Obika S., Nanbu,D., Hari,Y., Andoh,J., Morio,K., Doi,T. and Imanishi,T. (1998) Stability and structural features of the duplexes containing nucleoside analogues with a fixed N-type conformation, 2′-O,4′-C-methyleneribonucleosides. Tetrahedron Lett., 39, 5401–5404.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

