Transcriptional stimulation by the DNA binding protein Hap46/BAG-1M involves hsp70/hsc70 molecular chaperones
- PMID: 12682371
- PMCID: PMC153731
- DOI: 10.1093/nar/gkg303
Transcriptional stimulation by the DNA binding protein Hap46/BAG-1M involves hsp70/hsc70 molecular chaperones
Abstract
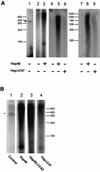
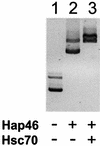
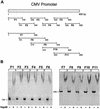
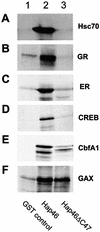
The hsp70/hsc70-associating protein Hap46 of human origin, also called BAG-1M (Bcl-2-associated athanogene 1), has been characterized previously as a DNA binding protein, which is able to stimulate transcription. By use of in vitro assays we now show that Hap46-mediated transcriptional activation can occur from linearized as well as from supercoiled circular DNA and does not require the presence of a transcription promoter. Accordingly, we observed no preferential binding of Hap46 to overlapping DNA fragments covering the sequence of the cytomegalovirus (CMV) early promoter, thus suggesting non-specific binding. The C-terminal deletion variant Hap46DeltaC47, which is unable to associate with hsp70/hsc70 molecular chaperones, produced greatly diminished effects on transcription, indicating a significant involvement of hsp70/hsc70 chaperones but not an absolute requirement. In contrast, deletion of the acidic hexarepeat region, as in variant Hap46Delta12-62, did not disturb transcriptional stimulation. While full-length Hap46 readily formed complexes with a series of structurally unrelated transcription factors, variant Hap46DeltaC47 proved incapable of doing so. Together these data suggest that transcriptional stimulation is a major biological activity of Hap46 and point to involvement of hsp70/hsc70 molecular chaperones in transcription in concert with Hap46, thus providing a link between hsp70/hsc70 molecular chaperones and components of the transcription machinery.
Figures


References
-
- Takayama S., Sato,T., Krajewski,S., Kochel,K., Irie,S., Millan,J.A. and Reed,J.C. (1995) Cloning and functional analysis of BAG-1: a novel Bcl-2-binding protein with anti-cell death activity. Cell, 80, 279–284. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

