In vivo antioxidant treatment protects against bleomycin-induced lung damage in rats
- PMID: 12684259
- PMCID: PMC1573750
- DOI: 10.1038/sj.bjp.0705138
In vivo antioxidant treatment protects against bleomycin-induced lung damage in rats
Abstract
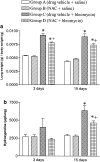
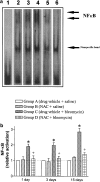
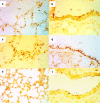
1. This study examines the activity of the antioxidant N-acetylcysteine on bleomycin-induced pulmonary fibrosis in rats with emphasis on the early inflammatory phase. 2. Rats receiving N-acetylcysteine (300 mg kg(-1) day(-1), intraperitoneal) had less augmented lung wet weight, and lower levels of proteins, lactate dehydrogenase, neutrophil and macrophage counts in bronchoalveolar lavage fluid and lung myeloperoxidase activity with a betterment of histological score at 3 days postbleomycin. 3. A diminished lung GSH/GSSG ratio and augmented lipid hydroperoxides were observed 3 days postbleomycin. These changes were attenuated by N-acetylcysteine. Alveolar macrophages from bleomycin-exposed rats released augmented amounts of superoxide anion and nitric oxide. N-Acetylcysteine did not modify superoxide anion generation but reduced the increased production of nitric oxide. 4. N-Acetylcysteine suppressed the bleomycin-induced increased activation of lung NF-kappaB (shift assay and immunohistochemistry), and decreased the augmented levels of the early inflammatory cytokines, tumour necrosis factor-alpha, interleukin-beta, interleukin-6 and macrophage inflammatory protein-2 observed in bronchoalveolar lavage fluid at 1 and 3 days postbleomycin exposure. 5. At 15 days postbleomycin, N-acetylcysteine decreased collagen deposition in bleomycin-exposed rats (hydroxyproline content: 6351+/-669 and 4626+/-288 micro g per lung in drug vehicle- and N-acetylcysteine-treated rats, respectively; P<0.05). Semiquantitative histological assessment at this stage showed less collagen deposition in N-acetylcysteine-treated rats compared to those receiving bleomycin alone. 6. These results indicate that N-acetylcysteine reduces the primary inflammatory events, thus preventing cellular damage and the subsequent development of pulmonary fibrosis in the bleomycin rat model.
Figures



Similar articles
-
Attenuation by oral N-acetylcysteine of bleomycin-induced lung injury in rats.Eur Respir J. 2001 Jun;17(6):1228-35. doi: 10.1183/09031936.01.00049701. Eur Respir J. 2001. PMID: 11491169
-
Diallyl sulfide attenuates bleomycin-induced pulmonary fibrosis: critical role of iNOS, NF-kappaB, TNF-alpha and IL-1beta.Life Sci. 2008 Jun 6;82(23-24):1142-53. doi: 10.1016/j.lfs.2008.03.018. Epub 2008 Apr 4. Life Sci. 2008. PMID: 18462759
-
Sinapic acid ameliorates bleomycin-induced lung fibrosis in rats.Biomed Pharmacother. 2018 Dec;108:224-231. doi: 10.1016/j.biopha.2018.09.032. Epub 2018 Sep 13. Biomed Pharmacother. 2018. PMID: 30219680
-
Use of tetrandrine to differentiate between mechanisms involved in silica-versus bleomycin-induced fibrosis.J Toxicol Environ Health A. 1999 Jun 25;57(4):247-66. doi: 10.1080/009841099157692. J Toxicol Environ Health A. 1999. PMID: 10406349
-
Thymoquinone blocks lung injury and fibrosis by attenuating bleomycin-induced oxidative stress and activation of nuclear factor Kappa-B in rats.Toxicology. 2012 Dec 16;302(2-3):106-13. doi: 10.1016/j.tox.2012.09.001. Epub 2012 Sep 12. Toxicology. 2012. PMID: 22982510
Cited by
-
Endothelial-to-mesenchymal transition in systemic sclerosis.Clin Exp Immunol. 2021 Jul;205(1):12-27. doi: 10.1111/cei.13599. Epub 2021 Apr 18. Clin Exp Immunol. 2021. PMID: 33772754 Free PMC article. Review.
-
Effect of resveratrol on treatment of bleomycin-induced pulmonary fibrosis in rats.Inflammation. 2012 Oct;35(5):1732-41. doi: 10.1007/s10753-012-9491-0. Inflammation. 2012. PMID: 22707284
-
A small molecule, orally active, alpha4beta1/alpha4beta7 dual antagonist reduces leukocyte infiltration and airway hyper-responsiveness in an experimental model of allergic asthma in Brown Norway rats.Br J Pharmacol. 2006 Mar;147(6):661-70. doi: 10.1038/sj.bjp.0706658. Br J Pharmacol. 2006. PMID: 16432509 Free PMC article.
-
Bach1 siRNA attenuates bleomycin-induced pulmonary fibrosis by modulating oxidative stress in mice.Int J Mol Med. 2017 Jan;39(1):91-100. doi: 10.3892/ijmm.2016.2823. Epub 2016 Dec 8. Int J Mol Med. 2017. PMID: 27959382 Free PMC article.
-
Triptolide mitigates radiation-induced pneumonitis via inhibition of alveolar macrophages and related inflammatory molecules.Oncotarget. 2017 Jul 11;8(28):45133-45142. doi: 10.18632/oncotarget.16456. Oncotarget. 2017. PMID: 28415830 Free PMC article.
References
-
- ALVAREZ A., SANZ M.J. Reactive oxygen species mediate angiotensin II-induced leukocyte–endothelial cell interactions in vivo. J. Leukoc. Biol. 2001;70:199–206. - PubMed
-
- ARUOMA O.I., HALLIWELL B., HOEY B.M., BUTLER J. The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic. Biol. Med. 1989;6:593–597. - PubMed
-
- ASENSI M., SASTRE J., PALLARDÓ J.V., GARCÍA DE LA ASUNCIÓN J., ESTRELA J.M., VIÑA J. A high-performance liquid chromatography method for measurement of oxidized glutathione in biological samples. Anal. Biochem. 1994;217:323–328. - PubMed
-
- BARNES P.J., KARIN M. Nuclear factor-κB—A pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 1997;336:1066–1071. - PubMed
-
- BEHR J., MAIER K., DEGENKOLB B., KROMBACH F., VOGELMEIR C. Antioxidative and clinical effects of high dose N-acetylcysteine in fibrosing alveolitis. Adjunctive therapy to maintenance immunosuppression. Am. J. Respir. Crit. Care Med. 1997;156:1897–1901. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

