Structural requirements for novel willardiine derivatives acting as AMPA and kainate receptor antagonists
- PMID: 12684265
- PMCID: PMC1573755
- DOI: 10.1038/sj.bjp.0705148
Structural requirements for novel willardiine derivatives acting as AMPA and kainate receptor antagonists
Abstract
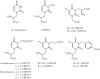
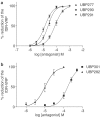
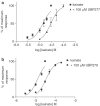
1. The natural product willardiine is an AMPA receptor agonist. We have examined the structural changes required to convert willardiine into an antagonist at AMPA and kainate receptors. Structure-activity analysis has been carried out to discover the structural features required to increase the potency and/or selectivity of the antagonists at AMPA or kainate receptors. 2. Reduction of the fast component of the dorsal root-evoked ventral root potential (fDR-VRP) has been used to investigate AMPA receptor antagonist activity. To examine antagonist activity at kainate receptors, the ability of compounds to depress kainate-induced depolarisations of dorsal root fibres was assessed. 3. Blocking ionisation of the uracil ring by adding a methyl group to the N(3) position was not sufficient to convert willardiine into an antagonist. However, willardiine derivatives with a side-chain bearing a carboxylic acid group at the N(3)-position of the uracil ring could antagonise AMPA and kainate receptors. 4. S stereochemistry was optimal for antagonism. When compounds with differing interacidic group chain lengths were compared, a group chain length of two methylene groups was preferable for AMPA receptor antagonism in the series of compounds bearing a carboxyalkyl side chain (UBP275, UBP277 and UBP279 reduced the fDR-VRP with IC(50) values of 287+/-41, 23.8+/-3.9 and 136+/-17 micro M, respectively). For kainate receptor antagonism, two or three methylene groups were almost equally acceptable (UBP277 and UBP279 reduced dorsal root kainate responses with apparent K(D) values of 73.1+/-4.5 and 60.5+/-4.1 micro M, respectively). 5. Adding an iodo group to the 5-position of UBP277 and UBP282 enhanced activity at kainate receptors (UBP291 and UBP301 antagonised kainate responses on the dorsal root with apparent K(D) values of 9.83+/-1.62 and 5.94+/-0.63 micro M, respectively). 6. The most useful antagonist identified in this study was UBP301, which was a potent and approximately 30-fold selective kainate receptor antagonist. UBP282 may also be of use in isolating a non-GluR5-mediated kainate response.
Figures
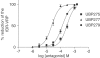
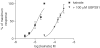
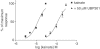
Similar articles
-
Willardiine and Its Synthetic Analogues: Biological Aspects and Implications in Peptide Chemistry of This Nucleobase Amino Acid.Pharmaceuticals (Basel). 2022 Oct 10;15(10):1243. doi: 10.3390/ph15101243. Pharmaceuticals (Basel). 2022. PMID: 36297355 Free PMC article. Review.
-
The novel antagonist 3-CBW discriminates between kainate receptors expressed on neonatal rat motoneurones and those on dorsal root C-fibres.Br J Pharmacol. 2002 Dec;137(7):1125-33. doi: 10.1038/sj.bjp.0704957. Br J Pharmacol. 2002. PMID: 12429586 Free PMC article.
-
Synthesis and pharmacology of willardiine derivatives acting as antagonists of kainate receptors.J Med Chem. 2005 Dec 1;48(24):7867-81. doi: 10.1021/jm050584l. J Med Chem. 2005. PMID: 16302825
-
Pharmacological differentiation of kainate receptors on neonatal rat spinal motoneurones and dorsal roots.Neuropharmacology. 1998 Oct-Nov;37(10-11):1223-37. doi: 10.1016/s0028-3908(98)00124-5. Neuropharmacology. 1998. PMID: 9849660
-
BGG492 (selurampanel), an AMPA/kainate receptor antagonist drug for epilepsy.Expert Opin Investig Drugs. 2014 Jan;23(1):107-13. doi: 10.1517/13543784.2014.848854. Epub 2013 Oct 23. Expert Opin Investig Drugs. 2014. PMID: 24147649 Review.
Cited by
-
Dancing with Nucleobases: Unveiling the Self-Assembly Properties of DNA and RNA Base-Containing Molecules for Gel Formation.Gels. 2023 Dec 23;10(1):16. doi: 10.3390/gels10010016. Gels. 2023. PMID: 38247739 Free PMC article. Review.
-
ACET is a highly potent and specific kainate receptor antagonist: characterisation and effects on hippocampal mossy fibre function.Neuropharmacology. 2009 Jan;56(1):121-30. doi: 10.1016/j.neuropharm.2008.08.016. Epub 2008 Aug 22. Neuropharmacology. 2009. PMID: 18789344 Free PMC article.
-
Willardiine and Its Synthetic Analogues: Biological Aspects and Implications in Peptide Chemistry of This Nucleobase Amino Acid.Pharmaceuticals (Basel). 2022 Oct 10;15(10):1243. doi: 10.3390/ph15101243. Pharmaceuticals (Basel). 2022. PMID: 36297355 Free PMC article. Review.
-
Kainate Receptor Antagonists: Recent Advances and Therapeutic Perspective.Int J Mol Sci. 2023 Jan 18;24(3):1908. doi: 10.3390/ijms24031908. Int J Mol Sci. 2023. PMID: 36768227 Free PMC article. Review.
-
Metabotropic glutamate receptor activity induces a novel oscillatory pattern in neonatal rat hypoglossal motoneurones.J Physiol. 2005 Feb 15;563(Pt 1):139-59. doi: 10.1113/jphysiol.2004.079509. Epub 2004 Dec 20. J Physiol. 2005. PMID: 15611018 Free PMC article.
References
-
- BETTLER B., BOULTER J., HERMANS-BORGMEYER I., O'SHEA-GREENFIELD A., DENERIS E.S., MOLL C., BORGMEYER U., HOLLMAN M, HEINEMANN S. Cloning of a novel glutamate receptor subunit GluR5: expression in the central nervous sysytem during development. Neuron. 1990;5:583–595. - PubMed
-
- BLEAKMAN D., BALLYK B.A., SCHOEPP D.D., PALMER A.J., BATH C.P., SHARPE E.F., WOOLLEY M.L., BUFTON H.R., KAMBOJ R.K., TARNAWA I., LODGE D. Activity of 2,3-benzodiazepines at native rat and recombinant human glutamate receptors in vitro: stereospecificity and selectivity profiles. Neuropharmacology. 1996;35:1689–1702. - PubMed
-
- BLEAKMAN D., LODGE D. Neuropharmacology of AMPA and kainate receptors. Neuropharmacology. 1998;37:1187–1204. - PubMed
-
- CHITTAJALLU R., BRAITHWAITE S.P., CLARKE V.R., HENLEY J.M. Kainate receptors: subunits, synaptic localization and function. Trends Pharmacol. Sci. 1999;20:26–35. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

