Transcriptional silencing of an amoebapore gene in Entamoeba histolytica: molecular analysis and effect on pathogenicity
- PMID: 12684379
- PMCID: PMC154849
- DOI: 10.1128/EC.2.2.295-305.2003
Transcriptional silencing of an amoebapore gene in Entamoeba histolytica: molecular analysis and effect on pathogenicity
Abstract
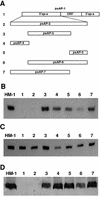
Transcriptional silencing of the gene coding for amoebapore A (AP-A) was observed when trophozoites of Entamoeba histolytica were transfected with a hybrid plasmid construct containing the ap-a gene flanked by the upstream and downstream segments of the original Ehap-a gene. Transfectants were totally devoid of ap-a transcript and AP-A protein. An identical silencing effect was observed upon transfection with a plasmid that contained only the 5' upstream region of ap-a. Removal of the selecting antibiotic enabled the isolation of plasmidless clones, which retained in their progeny the silenced phenotype. E. histolytica cells were able to overexpress ap-a when transfected with a plasmid containing the gene flanked by the 5' and 3' regions of the EhRP-L21 gene. This plasmid, however, could not express ap-a in the retransfected, cloned trophozoites lacking AP-A. This is the first report of gene silencing in E. histolytica, and the mechanism appears to belong to transcriptional gene silencing and not to posttranscriptional gene silencing. This conclusion is based on the following results: (i) silencing was achieved by transfection of homologous 5' flanking sequences (470 bp of the Ehap-a gene), (ii) transcription initiation of Ehap-a was found to be blocked, and (iii) short double-stranded RNA fragments of the ap-a coding and noncoding sequences were not detected. Trophozoites lacking AP-A are nonpathogenic and impaired in their bacteriolytic capability.
Figures





References
-
- Alon, R. N., R. Bracha, and D. Mirelman. 1997. Inhibition of expression of the lysine-rich 30 kDa surface antigen of Entamoeba dispar by the transcription of its antisense RNA. Mol. Biochem. Parasitol. 90:193-201. - PubMed
-
- Ankri, S., F. Padilla-Vaca, T. Stolarsky, L. Koole, U. Katz, and D. Mirelman. 1999. Antisense inhibition of expression of the light subunit (35 kDa) of the Gal/GalNAc lectin complex inhibits Entamoeba histolytica virulence. Mol. Microbiol. 33:327-337. - PubMed
-
- Ankri, S., T. Stolarsky, and D. Mirelman. 1998. Antisense inhibition of expression of cysteine proteinases in Entamoeba histolytica does not affect cytopathic or hemolytic activity but inhibits phagocytosis. Mol. Microbiol. 28:777-785. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

