The Hog1 mitogen-activated protein kinase is essential in the oxidative stress response and chlamydospore formation in Candida albicans
- PMID: 12684384
- PMCID: PMC154845
- DOI: 10.1128/EC.2.2.351-361.2003
The Hog1 mitogen-activated protein kinase is essential in the oxidative stress response and chlamydospore formation in Candida albicans
Abstract
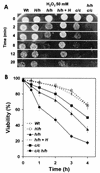
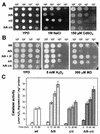
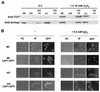
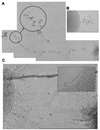
Candida albicans mutants with mutations in mitogen-activated protein (MAP) kinase HOG1 displayed an increased sensitivity to agents producing reactive oxygen species, such as oxidants (menadione, hydrogen peroxide, or potassium superoxide), and UV light. Consistent with this finding, C. albicans Hog1 was activated not only in response to an increase in external osmolarity, as happens with its Saccharomyces cerevisiae homologue, but also in response to hydrogen peroxide. The Hog1-mediated response to oxidative stress was different from that of transcription factor Cap1, the homologue of S. cerevisiae Yap1, as shown by the different sensitivities to oxidants and the kinetics of cell death of cap1Delta, hog1, and hog1 cap1Delta mutants. Deletion of CAP1 did not influence the level of Hog1 phosphorylation, and deletion of HOG1 did not affect Cap1 nuclear localization. Moreover, we show that the HOG1 gene plays a role in chlamydospore formation, another oxygen-related morphogenetic event, as demonstrated by the fact that hog1 cells were unable to generate these thick-walled structures in several media through a mechanism different from that of the EFG1 regulator. This is the first demonstration of the role of the Hog1-mediated MAP kinase pathway in resistance to oxidative stress in pathogenic fungi, and it allows us to propose a molecular model for the oxidative stress response in C. albicans.
Figures



References
-
- Alarco, A. M., I. Balan, D. Talibi, N. Mainville, and M. Raymond. 1997. AP1-mediated multidrug resistance in Saccharomyces cerevisiae requires FLR1 encoding a transporter of the major facilitator superfamily. J. Biol. Chem. 272:19304-19313. - PubMed
-
- Bossier, P., L. Fernandes, D. Rocha, and C. Rodrigues-Pousada. 1993. Overexpression of YAP2, coding for a new yAP protein, and YAP1 in Saccharomyces cerevisiae alleviates growth inhibition caused by 1,10-phenanthroline. J. Biol. Chem. 268:23640-23645. - PubMed
-
- Brewster, J. L., T. de Valoir, N. D. Dwyer, E. Winter, and M. C. Gustin. 1993. An osmosensing signal transduction pathway in yeast. Science 259:1760-1763. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

