Vasoactive intestinal polypeptide and pituitary adenylate cyclase-activating polypeptide activate hyperpolarization-activated cationic current and depolarize thalamocortical neurons in vitro
- PMID: 12684461
- PMCID: PMC6742061
- DOI: 10.1523/JNEUROSCI.23-07-02751.2003
Vasoactive intestinal polypeptide and pituitary adenylate cyclase-activating polypeptide activate hyperpolarization-activated cationic current and depolarize thalamocortical neurons in vitro
Abstract
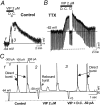
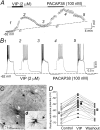
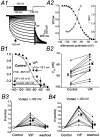
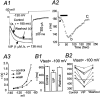
Ascending pathways mediated by monoamine neurotransmitters regulate the firing mode of thalamocortical neurons and modulate the state of brain activity. We hypothesized that specific neuropeptides might have similar actions. The effects of vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase-activating polypeptide (PACAP) were tested on thalamocortical neurons using whole-cell patch-clamp techniques applied to visualized neurons in rat brain slices. VIP (2 microm) and PACAP (100 nm) reversibly depolarized thalamocortical neurons (7.8 +/- 0.6 mV; n = 16), reduced the membrane resistance by 33 +/- 3%, and could convert the firing mode from bursting to tonic. These effects on resting membrane potential and membrane resistance persisted in the presence of TTX. Morphologically diverse thalamocortical neurons located in widespread regions of thalamus were all depolarized by VIP and PACAP38. In voltage-clamp mode, we found that VIP and PACAP38 reversibly activated a hyperpolarization-activated cationic current (I(H)) in thalamocortical neurons and altered voltage- and time-dependent activation properties of the current. The effects of VIP on membrane conductance were abolished by the hyperpolarization-activated cyclic-nucleotide-gated channel (HCN)-specific antagonist ZD7288, showing that HCN channels are the major target of VIP modulation. The effects of VIP and PACAP38 on HCN channels were mediated by PAC(1) receptors and cAMP. The actions of PACAP-related peptides on thalamocortical neurons suggest an additional and novel endogenous neurophysiological pathway that may influence both normal and pathophysiological thalamocortical rhythm generation and have important behavioral effects on sensory processing and sleep-wake cycles.
Figures


Similar articles
-
Facilitatory effects of pituitary adenylate cyclase activating polypeptide (PACAP) on neurons in the magnocellular portion of the rat hypothalamic paraventricular nucleus (PVN) in vitro.J Neuroendocrinol. 1996 Feb;8(2):137-43. doi: 10.1111/j.1365-2826.1996.tb00834.x. J Neuroendocrinol. 1996. PMID: 8868261
-
VPAC2-R mediates the lipolytic effects of pituitary adenylate cyclase-activating polypeptide/vasoactive intestinal polypeptide in primary rat adipocytes.Endocrinology. 2005 Feb;146(2):744-50. doi: 10.1210/en.2004-0504. Epub 2004 Oct 28. Endocrinology. 2005. PMID: 15514088
-
Pituitary adenylate cyclase-activating polypeptide and vasoactive intestinal peptide inhibit dendritic growth in cultured sympathetic neurons.J Neurosci. 2002 Aug 1;22(15):6560-9. doi: 10.1523/JNEUROSCI.22-15-06560.2002. J Neurosci. 2002. PMID: 12151535 Free PMC article.
-
Pituitary adenylate cyclase-activating polypeptides, PACAP-38 and PACAP-27, regulation of sympathetic neuron catecholamine, and neuropeptide Y expression through activation of type I PACAP/VIP receptor isoforms.Ann N Y Acad Sci. 1996 Dec 26;805:204-16; discussion 217-8. doi: 10.1111/j.1749-6632.1996.tb17484.x. Ann N Y Acad Sci. 1996. PMID: 8993404 Review.
-
PACAP/VIP receptors in pancreatic beta-cells: their roles in insulin secretion.Ann N Y Acad Sci. 1996 Dec 26;805:44-51; discussion 52-3. doi: 10.1111/j.1749-6632.1996.tb17472.x. Ann N Y Acad Sci. 1996. PMID: 8993392 Review.
Cited by
-
Regulation of recombinant and native hyperpolarization-activated cation channels.Mol Neurobiol. 2004 Dec;30(3):279-305. doi: 10.1385/MN:30:3:279. Mol Neurobiol. 2004. PMID: 15655253 Review.
-
Select cognitive deficits in vasoactive intestinal peptide deficient mice.BMC Neurosci. 2008 Jul 10;9:63. doi: 10.1186/1471-2202-9-63. BMC Neurosci. 2008. PMID: 18616823 Free PMC article.
-
Emerging pharmacological targets for alcohol use disorder.Alcohol. 2024 Dec;121:103-114. doi: 10.1016/j.alcohol.2024.07.007. Epub 2024 Jul 26. Alcohol. 2024. PMID: 39069210 Review.
-
Chronic valproic acid treatment triggers increased neuropeptide y expression and signaling in rat nucleus reticularis thalami.J Neurosci. 2006 Jun 21;26(25):6813-22. doi: 10.1523/JNEUROSCI.5320-05.2006. J Neurosci. 2006. PMID: 16793888 Free PMC article.
-
Variation in Activity State, Axonal Projection, and Position Define the Transcriptional Identity of Individual Neocortical Projection Neurons.Cell Rep. 2018 Jan 9;22(2):441-455. doi: 10.1016/j.celrep.2017.12.046. Cell Rep. 2018. PMID: 29320739 Free PMC article.
References
-
- Ahnaou A, Laporte AM, Ballet S, Escourrou P, Hamon M, Adrien J, Bourgin P. Muscarinic and PACAP receptor interactions at pontine level in the rat: significance for REM sleep regulation. Eur J Neurosci. 2000;12:4496–4504. - PubMed
-
- Bourgin P, Lebrand C, Escourrou P, Gaultier C, Franc B, Hamon M, Adrien J. Vasoactive intestinal polypeptide microinjections into the oral pontine tegmentum enhance rapid eye movement sleep in the rat. Neuroscience. 1997;77:351–360. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
