A chondroitin sulfate proteoglycan PTPzeta /RPTPbeta regulates the morphogenesis of Purkinje cell dendrites in the developing cerebellum
- PMID: 12684467
- PMCID: PMC6742081
- DOI: 10.1523/JNEUROSCI.23-07-02804.2003
A chondroitin sulfate proteoglycan PTPzeta /RPTPbeta regulates the morphogenesis of Purkinje cell dendrites in the developing cerebellum
Abstract
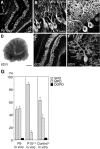
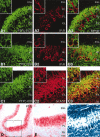
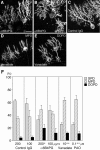
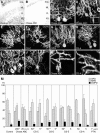
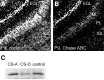
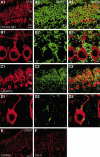
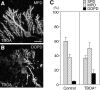
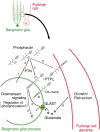
PTPzeta/RPTPbeta, a receptor-type protein tyrosine phosphatase synthesized as a chondroitin sulfate (CS) proteoglycan, uses a heparin-binding growth factor pleiotrophin (PTN) as a ligand, in which the CS portion plays an essential role in ligand binding. Using an organotypic slice culture system, we tested the hypothesis that PTN-PTPzeta signaling is involved in the morphogenesis of Purkinje cell dendrites. An aberrant morphology of Purkinje cell dendrites such as multiple and disoriented primary dendrites was induced in slice cultures by (1) addition of a polyclonal antibody against the extracellular domain of PTPzeta, (2) inhibition of protein tyrosine phosphatase activity, (3) enzymatic removal of the CS chains, (4) addition of exogenous CS chains, and (5) addition of exogenous PTN, all of which disturb PTN-PTPzeta signaling. These treatments also reduced the immunoreactivity to GLAST, a glial glutamate transporter, on Bergmann glial processes. Furthermore, a glutamate transporter inhibitor also induced the abnormal morphogenesis of Purkinje cell dendrites. Altogether, these findings suggest that PTN-PTPzeta signaling regulates the morphogenesis of Purkinje cell dendrites and that the mechanisms underlying that regulation involve the GLAST activity in Bergmann glial processes.
Figures

Similar articles
-
[Mechanisms for dendritic morphogenesis of cerebellar purkinje cells: role of receptor-type protein tyrosine phosphatase zeta].Nihon Shinkei Seishin Yakurigaku Zasshi. 2007 Jun;27(3):135-40. Nihon Shinkei Seishin Yakurigaku Zasshi. 2007. PMID: 17633525 Review. Japanese.
-
Involvement of receptor-like protein tyrosine phosphatase zeta/RPTPbeta and its ligand pleiotrophin/heparin-binding growth-associated molecule (HB-GAM) in neuronal migration.J Cell Biol. 1998 Jul 13;142(1):203-16. doi: 10.1083/jcb.142.1.203. J Cell Biol. 1998. PMID: 9660874 Free PMC article.
-
Spatio-temporal characterization of the pleiotrophinergic system in mouse cerebellum: evidence for its key role during ontogenesis.Exp Neurol. 2013 Sep;247:537-51. doi: 10.1016/j.expneurol.2013.02.004. Epub 2013 Feb 20. Exp Neurol. 2013. PMID: 23454176
-
6B4 proteoglycan/phosphacan, an extracellular variant of receptor-like protein-tyrosine phosphatase zeta/RPTPbeta, binds pleiotrophin/heparin-binding growth-associated molecule (HB-GAM).J Biol Chem. 1996 Aug 30;271(35):21446-52. doi: 10.1074/jbc.271.35.21446. J Biol Chem. 1996. PMID: 8702927
-
Anaplastic lymphoma kinase: "Ligand Independent Activation" mediated by the PTN/RPTPβ/ζ signaling pathway.Biochim Biophys Acta. 2013 Oct;1834(10):2219-23. doi: 10.1016/j.bbapap.2013.06.004. Epub 2013 Jun 15. Biochim Biophys Acta. 2013. PMID: 23777859 Review.
Cited by
-
The Dendritic Differentiation of Purkinje Neurons: Unsolved Mystery in Formation of Unique Dendrites.Cerebellum. 2015 Jun;14(3):227-30. doi: 10.1007/s12311-014-0585-0. Cerebellum. 2015. PMID: 25015299 No abstract available.
-
Receptor protein tyrosine phosphatase beta/zeta is a functional binding partner for vascular endothelial growth factor.Mol Cancer. 2015 Feb 3;14(1):19. doi: 10.1186/s12943-015-0287-3. Mol Cancer. 2015. PMID: 25644401 Free PMC article.
-
Degradation of chondroitin sulfate proteoglycans induces sprouting of intact purkinje axons in the cerebellum of the adult rat.J Neurosci. 2005 Aug 3;25(31):7150-8. doi: 10.1523/JNEUROSCI.0683-05.2005. J Neurosci. 2005. PMID: 16079397 Free PMC article.
-
Phosphacan and receptor protein tyrosine phosphatase β expression mediates deafferentation-induced synaptogenesis.Hippocampus. 2011 Jan;21(1):81-92. doi: 10.1002/hipo.20725. Hippocampus. 2011. PMID: 20014386 Free PMC article.
-
Astrocyte-derived PTPRZ1 regulates astrocyte morphology and excitatory synaptogenesis.bioRxiv [Preprint]. 2025 May 1:2025.04.30.651514. doi: 10.1101/2025.04.30.651514. bioRxiv. 2025. PMID: 40654782 Free PMC article. Preprint.
References
-
- Altman J, Anderson WJ. Experimental reorganization of the cerebellar cortex. I. Morphological effects of eliminating all microneurons with prolonged X-irradiation started at birth. J Comp Neurol. 1972;146:355–406. - PubMed
-
- Amet LEA, Lauri SE, Hienola A, Croll SD, Lu Y, Levorse JM, Prabhakaran B, Taira T, Rauvala H, Vogt TF. Enhanced hippocampal long-term potentiation in mice lacking heparin-binding growth-associated molecule. Mol Cell Neurosci. 2001;17:1014–1024. - PubMed
-
- Armengol J-A, Sotelo C. Early dendritic development of Purkinje cells in the rat cerebellum. A light and electron microscopic study using axonal tracing in “in vitro” slices. Dev Brain Res. 1991;64:95–114. - PubMed
-
- Bandtlow CE, Zimmermann DR. Proteoglycans in the developing brain: new conceptual insights for old proteins. Physiol Rev. 2000;80:1267–1290. - PubMed
-
- Baptista CA, Hatten ME, Blazeski R, Mason CA. Cell-cell interactions influence survival and differentiation of purified Purkinje cells in vitro. Neuron. 1994;12:243–260. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
