The predominant neural stem cell isolated from postnatal and adult forebrain but not early embryonic forebrain expresses GFAP
- PMID: 12684469
- PMCID: PMC6742109
- DOI: 10.1523/JNEUROSCI.23-07-02824.2003
The predominant neural stem cell isolated from postnatal and adult forebrain but not early embryonic forebrain expresses GFAP
Abstract
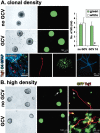
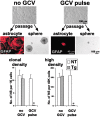
Periventricular germinal zones (GZs) of developing and adult brain contain neural stem cells (NSCs), the cellular identities and origins of which are not defined completely. We used tissue culture techniques and transgenic mice expressing herpes simplex virus thymidine kinase (HSV-TK) from the mouse glial fibrillary acid protein (GFAP) promoter to test the hypothesis that certain NSCs express GFAP. To do so, we determined the relative proportions of multipotent neurospheres that are formed by GFAP-expressing cells derived from GZs at different stages of development. In this transgenic model, dividing GFAP-expressing cells are ablated selectively by treatment with the antiviral agent ganciclovir (GCV). Single-cell analysis showed that transgene-derived HSV-TK was present only in GFAP-expressing cells. GCV applied in vitro eliminated growth of multipotent neurospheres from GZs of postnatal and adult transgenic mice but not early embryonic (embryonic day 12.5) transgenic mice. GCV prevented growth of secondary multipotent neurospheres prepared after passage of primary transgenic neurospheres derived from all three of these developmental stages. In addition, GCV prevented growth of multipotent neurospheres from transgenic astrocyte-enriched cell cultures derived from postnatal GZ, and elaidic acid GCV given for 4 d to adult transgenic mice in vivo abolished the ability to grow multipotent neurospheres from GZ. Extensive control experiments, including clonal analysis, demonstrated that failure of neurosphere growth was not merely secondary to loss of GFAP-expressing support cells or the result of a nonspecific toxic effect. Our findings demonstrate that the predominant multipotent NSCs isolated from postnatal and adult but not early embryonic GZs express GFAP, and that NSCs exhibit heterogeneous expression of intermediate filaments during developmental maturation.
Figures



References
-
- Alvarez-Buylla A, Garcia-Verdugo JM, Tramontin AD. A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci. 2001;2:287–293. - PubMed
-
- Anderson DJ. Stem cells and pattern formation in the nervous system: the possible versus the actual. Neuron. 2001;30:19–35. - PubMed
-
- Andrae J, Bongcam-Rudloff E, Hansson I, Lendahl U, Westermark B, Nister M. A 1.8 kb GFAP-promoter fragment is active in specific regions of the embryonic CNS. Mech Dev. 2001;107:181–185. - PubMed
-
- Balzarini J, Degreve B, Andrei G, Neyts J, Sandvold M, Myhren F, de Clercq E. Superior cytostatic activity of the ganciclovir elaidic acid ester due to the prolonged intracellular retention of ganciclovir anabolites in herpes simplex virus type 1 thymidine kinase gene-transfected tumor cells. Gene Ther. 1998;5:419–426. - PubMed
-
- Bush TG, Savidge TC, Freeman TC, Cox HJ, Campbell EA, Mucke L, Johnson MH, Sofroniew MV. Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell. 1998;93:189–201. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
