Transmission security for single kinesthetic afferent fibers of joint origin and their target cuneate neurons in the cat
- PMID: 12684485
- PMCID: PMC6742091
- DOI: 10.1523/JNEUROSCI.23-07-02980.2003
Transmission security for single kinesthetic afferent fibers of joint origin and their target cuneate neurons in the cat
Abstract
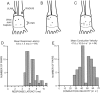
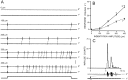
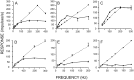
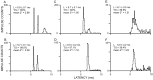
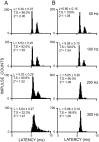
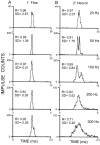
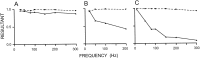
Transmission between single identified, kinesthetic afferent fibers of joint origin and their central target neurons of the cuneate nucleus was examined in anesthetized cats by means of paired electrophysiological recording. Fifty-three wrist joint afferent-cuneate neuron pairs were isolated in which the single joint afferent fiber exerted suprathreshold excitatory actions on the target cuneate neuron. For each pair, the minimum kinesthetic input, a single spike, was sufficient to generate cuneate spike output, often amplified as a pair or burst of spikes, particularly at input rates up to 50-100 impulses per second. The high security was confirmed quantitatively by construction of stimulus-response relationships and calculation of transmission security measures in response to both static and dynamic vibrokinesthetic disturbances applied to the joint capsule. Graded stimulus-response relationships demonstrated that the output for this synaptic connection between single joint afferents and cuneate neurons could provide a sensitive indicator of the strength of joint capsule stimuli. The transmission security measures, calculated as the proportion of joint afferent spikes that generated cuneate spike output, were high (>85-90%) even at afferent fiber discharge rates up to 100-200 impulses per second. Furthermore, tight phase locking in the cuneate responses to vibratory stimulation of the joint capsule demonstrated that the synaptic linkage preserved, with a high level of fidelity, the temporal information about dynamic kinesthetic perturbations that affected the joint. The present study establishes that single kinesthetic afferents of joint origin display a capacity similar to that of tactile afferent fibers for exerting potent synaptic actions on central target neurons of the major ascending kinesthetic sensory pathway.
Figures


Similar articles
-
Transmission security for single, hair follicle-related tactile afferent fibers and their target cuneate neurons in cat.J Neurophysiol. 2001 Aug;86(2):900-11. doi: 10.1152/jn.2001.86.2.900. J Neurophysiol. 2001. PMID: 11495959
-
Synaptic transmission between single slowly adapting type I fibres and their cuneate target neurones in cat.J Physiol. 1994 Feb 1;474(3):379-92. doi: 10.1113/jphysiol.1994.sp020030. J Physiol. 1994. PMID: 8014900 Free PMC article.
-
Transmission characteristics for the 1:1 linkage between slowly adapting type II fibers and their cuneate target neurons in cat.Exp Brain Res. 1995;105(1):67-75. doi: 10.1007/BF00242183. Exp Brain Res. 1995. PMID: 7589319
-
Synaptic transmission between single tactile and kinaesthetic sensory nerve fibers and their central target neurones.Behav Brain Res. 2002 Sep 20;135(1-2):197-212. doi: 10.1016/s0166-4328(02)00166-3. Behav Brain Res. 2002. PMID: 12356451 Review.
-
The synaptic linkage for tactile and kinaesthetic inputs to the dorsal column nuclei.Adv Exp Med Biol. 2002;508:47-55. doi: 10.1007/978-1-4615-0713-0_7. Adv Exp Med Biol. 2002. PMID: 12171144 Review.
Cited by
-
Impairment of human proprioception by high-frequency cutaneous vibration.J Physiol. 2007 Jun 15;581(Pt 3):971-80. doi: 10.1113/jphysiol.2006.126854. Epub 2007 Apr 5. J Physiol. 2007. PMID: 17412774 Free PMC article.
-
Impulse propagation over tactile and kinaesthetic sensory axons to central target neurones of the cuneate nucleus in cat.J Physiol. 2003 Jul 15;550(Pt 2):553-62. doi: 10.1113/jphysiol.2002.037002. Epub 2003 May 23. J Physiol. 2003. PMID: 12766249 Free PMC article.
-
Tonic and phasic differential GABAergic inhibition of synaptic actions of joint afferents in the cat.Exp Brain Res. 2007 Jan;176(1):98-118. doi: 10.1007/s00221-006-0600-x. Epub 2006 Aug 1. Exp Brain Res. 2007. PMID: 16896983
-
Changes in synaptic effectiveness of myelinated joint afferents during capsaicin-induced inflammation of the footpad in the anesthetized cat.Exp Brain Res. 2008 May;187(1):71-84. doi: 10.1007/s00221-008-1281-4. Epub 2008 Feb 5. Exp Brain Res. 2008. PMID: 18251018
-
Neuronal mechanisms mediating the variability of somatosensory evoked potentials during sleep oscillations in cats.J Physiol. 2005 Jan 15;562(Pt 2):569-82. doi: 10.1113/jphysiol.2004.071381. Epub 2004 Nov 4. J Physiol. 2005. PMID: 15528249 Free PMC article.
References
-
- Andersen P, Eccles JC, Schmidt RF, Yokota T. Identification of relay cells and interneurons in the cuneate nucleus. J Neurophysiol. 1964;27:1085–1095. - PubMed
-
- Asanuma H, Zarzecki P, Jankowska E, Hongo T, Marcus S. Projection of individual pyramidal tract neurons to lumbar motor nuclei of the monkey. Exp Brain Res. 1979;34:73–89. - PubMed
-
- Baxendale RH, Ferrell WR, Wood L. Responses of quadriceps motor units to mechanical stimulation of knee joint receptors in the decerebrate cat. Brain Res. 1988;453:150–156. - PubMed
-
- Berkley KJ, Budell RJ, Blomqvist A, Bull M. Output systems of the dorsal column nuclei in the cat. Brain Res. 1986;396:199–225. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous
