Directional avoidance turns encoded by single interneurons and sustained by multifunctional serotonergic cells
- PMID: 12684491
- PMCID: PMC6742103
- DOI: 10.1523/JNEUROSCI.23-07-03039.2003
Directional avoidance turns encoded by single interneurons and sustained by multifunctional serotonergic cells
Abstract
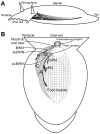
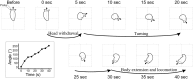
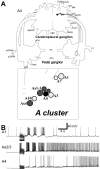
Avoidance turns in the sea slug Pleurobranchaea are responses to noxious stimuli and replace orienting turns to food stimuli after avoidance conditioning or satiation. Avoidance turns proved to be centrally patterned behaviors, the fictive expression of which could be elicited in reduced preparations and the isolated CNS. Activity in one of a bilateral interneuron pair, the A4 cells, was necessary and sufficient to drive the avoidance turn toward the contralateral side. Single A4 cells appeared to encode both turn direction and angle, in contrast to directional behaviors of other animals in which displacement angle is usually encoded by multiple units. The As1-4 cells, bilateral serotonergic cell clusters, excited the prolonged A4 burst during the turn through electrical and chemical coupling. However, during the escape swim, As1-4 became integral elements of the swim motor network, and A4 activity was entrained to the swim rhythm by alternating excitatory-inhibitory inputs, with only weak spiking. This provides a likely mechanism for the previously observed suppression of the avoidance turn by escape swimming. These observations add significant new aspects to the multiplying known functions of As1-4 and their homologs in other molluscs and point to a pivotal role of these neurons in the organization of gastropod behavior. Simple functional models predict (1) the essential actions of inhibitor neurons in the directionality of the turning network motor output and (2) an integrating role for As1-4 in the behavioral switch between turning avoidance and swimming escape, on the basis of their response to increasing stimulus intensity.
Figures











Similar articles
-
Escape swim network interneurons have diverse roles in behavioral switching and putative arousal in Pleurobranchaea.J Neurophysiol. 2000 Mar;83(3):1346-55. doi: 10.1152/jn.2000.83.3.1346. J Neurophysiol. 2000. PMID: 10712462
-
Central pattern generator for escape swimming in the notaspid sea slug Pleurobranchaea californica.J Neurophysiol. 1999 Feb;81(2):654-67. doi: 10.1152/jn.1999.81.2.654. J Neurophysiol. 1999. PMID: 10036268
-
Neuronal elements that mediate escape swimming and suppress feeding behavior in the predatory sea slug Pleurobranchaea.J Neurophysiol. 1995 Nov;74(5):1900-10. doi: 10.1152/jn.1995.74.5.1900. J Neurophysiol. 1995. PMID: 8592183
-
Neuromodulation intrinsic to the central pattern generator for escape swimming in Tritonia.Ann N Y Acad Sci. 1998 Nov 16;860:181-8. doi: 10.1111/j.1749-6632.1998.tb09048.x. Ann N Y Acad Sci. 1998. PMID: 9928311 Review.
-
Modulation of swimming speed in the pteropod mollusc, Clione limacina: role of a compartmental serotonergic system.Invert Neurosci. 1996 Dec;2(3):157-65. doi: 10.1007/BF02214171. Invert Neurosci. 1996. PMID: 9372161 Review.
Cited by
-
A core circuit module for cost/benefit decision.Front Neurosci. 2012 Aug 31;6:123. doi: 10.3389/fnins.2012.00123. eCollection 2012. Front Neurosci. 2012. PMID: 22969700 Free PMC article.
-
An input-representing interneuron regulates spike timing and thereby phase switching in a motor network.J Neurosci. 2008 Feb 20;28(8):1916-28. doi: 10.1523/JNEUROSCI.4755-07.2008. J Neurosci. 2008. PMID: 18287508 Free PMC article.
-
Variations on a theme: species differences in synaptic connectivity do not predict central pattern generator activity.J Neurophysiol. 2017 Aug 1;118(2):1123-1132. doi: 10.1152/jn.00203.2017. Epub 2017 May 24. J Neurophysiol. 2017. PMID: 28539397 Free PMC article.
-
Variable neuronal participation in stereotypic motor programs.PLoS One. 2012;7(7):e40579. doi: 10.1371/journal.pone.0040579. Epub 2012 Jul 16. PLoS One. 2012. PMID: 22815768 Free PMC article.
-
A conserved gastropod withdrawal circuit in Biomphalaria glabrata, an intermediate host for schistosomiasis.J Neurophysiol. 2024 May 1;131(5):903-913. doi: 10.1152/jn.00390.2023. Epub 2024 Mar 13. J Neurophysiol. 2024. PMID: 38478883 Free PMC article.
References
-
- Bablanian GM, Weiss KR, Kupfermann I. Motor control of the appetitive phase of feeding behavior in Aplysia. Behav Neural Biol. 1987;48:394–407. - PubMed
-
- Brace RC. Shell attachment and associated musculature in the Notaspidea and Anaspidea (Gastropoda: Opisthobranchia). Trans Zool Soc Lond. 1977a;34:31–42.
-
- Brace RC. The functional anatomy of the mantle complex and columellar muscle of tectibranch molluscs (Gastropoda: Opisthobranchia), and its bearing on the evolution of opisthobranch organization. Philos Trans R Soc Lond B Biol Sci. 1977b;277:1–54. - PubMed
-
- Comer CM, Robertson RM. Identified nerve cells and insect behavior. Prog Neurobiol. 2001;63:409–439. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
