Extracellular signal-regulated kinase mediates phosphorylation of tropomyosin-1 to promote cytoskeleton remodeling in response to oxidative stress: impact on membrane blebbing
- PMID: 12686598
- PMCID: PMC153111
- DOI: 10.1091/mbc.e02-04-0235
Extracellular signal-regulated kinase mediates phosphorylation of tropomyosin-1 to promote cytoskeleton remodeling in response to oxidative stress: impact on membrane blebbing
Abstract
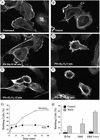
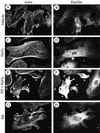
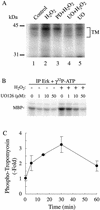
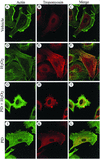
Oxidative stress induces in endothelial cells a quick and transient coactivation of both stress-activated protein kinase-2/p38 and extracellular signal-regulated kinase (ERK) mitogen-activated protein kinases. We found that inhibiting the ERK pathway resulted, within 5 min of oxidative stress, in a misassembly of focal adhesions characterized by mislocalization of key proteins such as paxillin. The focal adhesion misassembly that followed ERK inhibition with the mitogen-activated protein kinase kinase (MEK) inhibitor PD098059 (2'-amino-3'-methoxyflavone) or with a kinase negative mutant of ERK in the presence of H(2)O(2) resulted in a quick and intense membrane blebbing that was associated with important damage to the endothelium. We isolated by two-dimensional gel electrophoresis a PD098059-sensitive phosphoprotein of 38 kDa that we identified, by mass spectrometry, as tropomyosin-1. In fact, H(2)O(2) induced a time-dependent phosphorylation of tropomyosin that was sensitive to inhibition by PD098059 and UO126 (1,4-diamino-2,3-dicyano-1,4-bis[2-aminophenylthio]butanediane). Tropomyosin phosphorylation was also induced by expression of a constitutively activated form of MEK1 (MEK(CA)), which confirms that its phosphorylation resulted from the activation of ERK. In unstimulated cells, tropomyosin-1 was found diffuse in the cells, whereas it quickly colocalized with actin and stress fibers upon stimulation of ERK by H(2)O(2) or by expression of MEK(CA). We propose that phosphorylation of tropomyosin-1 downstream of ERK by contributing to formation of actin filaments increases cellular contractility and promotes the formation of focal adhesions. Incidentally, ML-7 (1-[5iodonaphthalene-1-sulfonyl]homopiperazine, HCl), an inhibitor of cell contractility, inhibited phosphorylation of tropomyosin and blocked the formation of stress fibers and focal adhesions, which also led to membrane blebbing in the presence of oxidative stress. Our finding that tropomyosin-1 is phosphorylated downstream of ERK, an event that modulates its interaction with actin, may lead to further understanding of the role of this protein in regulating cellular functions associated with cytoskeletal remodeling.
Figures




Similar articles
-
DAP kinase mediates the phosphorylation of tropomyosin-1 downstream of the ERK pathway, which regulates the formation of stress fibers in response to oxidative stress.J Cell Sci. 2007 Oct 15;120(Pt 20):3666-77. doi: 10.1242/jcs.003251. Epub 2007 Sep 25. J Cell Sci. 2007. PMID: 17895359
-
SAPK2/p38-dependent F-actin reorganization regulates early membrane blebbing during stress-induced apoptosis.J Cell Biol. 1998 Nov 30;143(5):1361-73. doi: 10.1083/jcb.143.5.1361. J Cell Biol. 1998. PMID: 9832563 Free PMC article.
-
The tumor vascular targeting agent combretastatin A-4-phosphate induces reorganization of the actin cytoskeleton and early membrane blebbing in human endothelial cells.Blood. 2002 Mar 15;99(6):2060-9. doi: 10.1182/blood.v99.6.2060. Blood. 2002. PMID: 11877280
-
Dysregulation of the endothelial cellular response to oxidative stress in cancer.Mol Carcinog. 2006 Jun;45(6):362-7. doi: 10.1002/mc.20218. Mol Carcinog. 2006. PMID: 16637066 Review.
-
The actin cytoskeleton response to oxidants: from small heat shock protein phosphorylation to changes in the redox state of actin itself.Free Radic Biol Med. 2001 Dec 15;31(12):1624-32. doi: 10.1016/s0891-5849(01)00749-3. Free Radic Biol Med. 2001. PMID: 11744337 Review.
Cited by
-
Characterization of the endothelial cell cytoskeleton following HLA class I ligation.PLoS One. 2012;7(1):e29472. doi: 10.1371/journal.pone.0029472. Epub 2012 Jan 11. PLoS One. 2012. PMID: 22247778 Free PMC article.
-
Regulation of cell migration and survival by focal adhesion targeting of Lasp-1.J Cell Biol. 2004 May 10;165(3):421-32. doi: 10.1083/jcb.200311045. J Cell Biol. 2004. PMID: 15138294 Free PMC article.
-
MEK modulates force-fluctuation-induced relengthening of canine tracheal smooth muscle.Eur Respir J. 2010 Sep;36(3):630-7. doi: 10.1183/09031936.00160209. Epub 2010 Jan 28. Eur Respir J. 2010. PMID: 20110395 Free PMC article.
-
Tropomyosin isoforms and reagents.Bioarchitecture. 2011 Jul;1(4):135-164. doi: 10.4161/bioa.1.4.17897. Epub 2011 Jul 1. Bioarchitecture. 2011. PMID: 22069507 Free PMC article.
-
Phosphorylation of actopaxin regulates cell spreading and migration.J Cell Biol. 2004 Sep 13;166(6):901-12. doi: 10.1083/jcb.200404024. Epub 2004 Sep 7. J Cell Biol. 2004. PMID: 15353548 Free PMC article.
References
-
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J Biol Chem. 1996;271:20246–20249. - PubMed
-
- Becker LC, Ambrosio G. Myocardial consequences of reperfusion. Prog Cardiovasc Dis. 1987;30:23–44. - PubMed
-
- Coleman ML, Sahai EA, Yeo M, Bosch M, Dewar A, Olson MF. Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat Cell Biol. 2001;3:339–345. - PubMed
-
- Cooper JA. Actin dynamics: tropomyosin provides stability. Curr Biol. 2002;12:R523–R525. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

