Autoinhibition of Jak2 tyrosine kinase is dependent on specific regions in its pseudokinase domain
- PMID: 12686600
- PMCID: PMC153113
- DOI: 10.1091/mbc.e02-06-0342
Autoinhibition of Jak2 tyrosine kinase is dependent on specific regions in its pseudokinase domain
Abstract
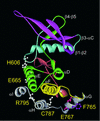
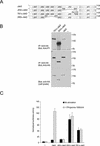
Jak tyrosine kinases have a unique domain structure containing a kinase domain (JH1) adjacent to a catalytically inactive pseudokinase domain (JH2). JH2 is crucial for inhibition of basal Jak activity, but the mechanism of this regulation has remained elusive. We show that JH2 negatively regulated Jak2 in bacterial cells, indicating that regulation is an intrinsic property of Jak2. JH2 suppressed basal Jak2 activity by lowering the V(max) of Jak2, whereas JH2 did not affect the K(m) of Jak2 for a peptide substrate. Three inhibitory regions (IR1-3) within JH2 were identified. IR3 (residues 758-807), at the C terminus of JH2, directly inhibited JH1, suggesting an inhibitory interaction between IR3 and JH1. Molecular modeling of JH2 showed that IR3 could form a stable alpha-helical fold, supporting that IR3 could independently inhibit JH1. IR2 (725-757) in the C-terminal lobe of JH2, and IR1 (619-670), extending from the N-terminal to the C-terminal lobe, enhanced IR3-mediated inhibition of JH1. Disruption of IR3 either by mutations or a small deletion increased basal Jak2 activity, but abolished interferon-gamma-inducible signaling. Together, the results provide evidence for autoinhibition of a Jak family kinase and identify JH2 regions important for autoregulation of Jak2.
Figures






References
-
- Abola EE, Sussman JL, Prilusky J, Manning NO. Protein Data Bank archives of three-dimensional macromolecular structures. Methods Enzymol. 1997;277:556–571. - PubMed
-
- Andreotti AH, Bunnell SC, Feng S, Berg LJ, Schreiber SL. Regulatory intramolecular association in a tyrosine kinase of the Tec family. Nature. 1997;385:93–97. - PubMed
-
- Candotti F, et al. Structural and functional basis for JAK3-deficient severe combined immunodeficiency. Blood. 1997;90:3996–4003. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

