Morphology and dynamics of clathrin/GGA1-coated carriers budding from the trans-Golgi network
- PMID: 12686608
- PMCID: PMC153121
- DOI: 10.1091/mbc.02-07-0109
Morphology and dynamics of clathrin/GGA1-coated carriers budding from the trans-Golgi network
Abstract
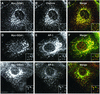
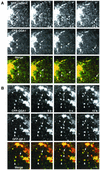
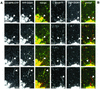
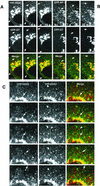
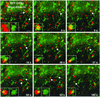
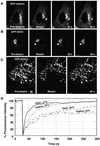
Sorting of transmembrane proteins and their ligands at various compartments of the endocytic and secretory pathways is mediated by selective incorporation into clathrin-coated intermediates. Previous morphological and biochemical studies have shown that these clathrin-coated intermediates consist of spherical vesicles with a diameter of 60-100 nm. Herein, we report the use of fluorescent imaging of live cells to demonstrate the existence of a different type of transport intermediate containing associated clathrin coats. Clathrin and the adaptors GGA1 and adaptor protein-1, labeled with different spectral variants of the green fluorescent protein, are shown to colocalize to the trans-Golgi network and to a population of vesicles and tubules budding from it. These intermediates are highly pleiomorphic and move toward the peripheral cytoplasm for distances of up to 10 microm with average speeds of approximately 1 microm/s. The labeled clathrin and GGA1 cycle on and off membranes with half-times of 10-20 s, independently of vesicle budding. Our observations indicate the existence of a novel type of trans-Golgi network-derived carriers containing associated clathrin, GGA1 and adaptor protein-1 that are larger than conventional clathrin-coated vesicles, and that undergo long-range translocation in the cytoplasm before losing their coats.
Figures


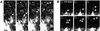
Similar articles
-
Cooperation of GGAs and AP-1 in packaging MPRs at the trans-Golgi network.Science. 2002 Sep 6;297(5587):1700-3. doi: 10.1126/science.1075327. Science. 2002. PMID: 12215646
-
Adaptor and clathrin exchange at the plasma membrane and trans-Golgi network.Mol Biol Cell. 2003 Feb;14(2):516-28. doi: 10.1091/mbc.e02-06-0353. Mol Biol Cell. 2003. PMID: 12589051 Free PMC article.
-
Structural basis for binding of accessory proteins by the appendage domain of GGAs.Nat Struct Biol. 2003 Aug;10(8):607-13. doi: 10.1038/nsb955. Nat Struct Biol. 2003. PMID: 12858163
-
Domains of the TGN: coats, tethers and G proteins.Traffic. 2004 May;5(5):315-26. doi: 10.1111/j.1398-9219.2004.00182.x. Traffic. 2004. PMID: 15086781 Review.
-
Visualization of TGN-endosome trafficking in mammalian and Drosophila cells.Methods Enzymol. 2012;504:255-71. doi: 10.1016/B978-0-12-391857-4.00013-6. Methods Enzymol. 2012. PMID: 22264539 Review.
Cited by
-
Regulation of the Golgi complex by phospholipid remodeling enzymes.Biochim Biophys Acta. 2012 Aug;1821(8):1078-88. doi: 10.1016/j.bbalip.2012.04.004. Epub 2012 Apr 22. Biochim Biophys Acta. 2012. PMID: 22562055 Free PMC article. Review.
-
Crystal structure of the clathrin adaptor protein 1 core.Proc Natl Acad Sci U S A. 2004 Sep 28;101(39):14108-13. doi: 10.1073/pnas.0406102101. Epub 2004 Sep 17. Proc Natl Acad Sci U S A. 2004. PMID: 15377783 Free PMC article.
-
Functional roles of short sequence motifs in the endocytosis of membrane receptors.Front Biosci (Landmark Ed). 2009 Jun 1;14(14):5339-60. doi: 10.2741/3599. Front Biosci (Landmark Ed). 2009. PMID: 19482617 Free PMC article. Review.
-
Epidermal growth factor-dependent phosphorylation of the GGA3 adaptor protein regulates its recruitment to membranes.Mol Cell Biol. 2005 Sep;25(18):7988-8000. doi: 10.1128/MCB.25.18.7988-8000.2005. Mol Cell Biol. 2005. PMID: 16135791 Free PMC article.
-
GGA function is required for maturation of neuroendocrine secretory granules.EMBO J. 2006 Apr 19;25(8):1590-602. doi: 10.1038/sj.emboj.7601067. Epub 2006 Apr 6. EMBO J. 2006. PMID: 16601685 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

