The crystal structure of dipeptidyl peptidase IV (CD26) reveals its functional regulation and enzymatic mechanism
- PMID: 12690074
- PMCID: PMC154298
- DOI: 10.1073/pnas.0230620100
The crystal structure of dipeptidyl peptidase IV (CD26) reveals its functional regulation and enzymatic mechanism
Abstract
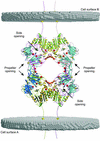
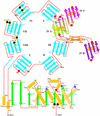
The membrane-bound glycoprotein dipeptidyl peptidase IV (DP IV, CD26) is a unique multifunctional protein, acting as receptor, binding and proteolytic molecule. We have determined the sequence and 1.8 A crystal structure of native DP IV prepared from porcine kidney. The crystal structure reveals a 2-2-2 symmetric tetrameric assembly which depends on the natively glycosylated beta-propeller blade IV. The crystal structure indicates that tetramerization of DP IV is a key mechanism to regulate its interaction with other components. Each subunit comprises two structural domains, the N-terminal eight-bladed beta-propeller with open Velcro topology and the C-terminal alpha/beta-hydrolase domain. Analogy with the structurally related POP and tricorn protease suggests that substrates access the buried active site through the beta-propeller tunnel while products leave the active site through a separate side exit. A dipeptide mimicking inhibitor complexed to the active site discloses key determinants for substrate recognition, including a Glu-Glu motif that distinguishes DP IV as an aminopeptidase and an oxyanion trap that binds and activates the P(2)-carbonyl oxygen necessary for efficient postproline cleavage. We discuss active and nonactive site-directed inhibition strategies of this pharmaceutical target protein.
Figures


References
-
- Yaron A, Naider F. Crit Rev Biochem Mol Biol. 1993;28:31–81. - PubMed
-
- Fleischer B. Immunol Today. 1994;15:180–184. - PubMed
-
- Kameoka J, Tanaka T, Nojima Y, Schlossman S F, Morimoto C. Science. 1993;261:466–469. - PubMed
-
- Dukecohan J S, Morimoto C, Rocker J A, Schlossman S F. J Immunol. 1996;165:1714–1721. - PubMed
-
- Lambeir A M, Pereira J F D, Chacon P, Vermeulen G, Heremans K, Devreese B, VanBeeumen J, Demeester I, Scharpe S. Biochim Biophys Acta. 1997;1340:215–226. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

