Acanthamoeba spp. as agents of disease in humans
- PMID: 12692099
- PMCID: PMC153146
- DOI: 10.1128/CMR.16.2.273-307.2003
Acanthamoeba spp. as agents of disease in humans
Abstract
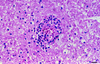
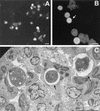
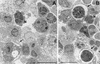
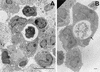
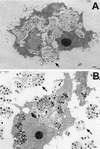
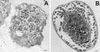
Acanthamoeba spp. are free-living amebae that inhabit a variety of air, soil, and water environments. However, these amebae can also act as opportunistic as well as nonopportunistic pathogens. They are the causative agents of granulomatous amebic encephalitis and amebic keratitis and have been associated with cutaneous lesions and sinusitis. Immuno compromised individuals, including AIDS patients, are particularly susceptible to infections with Acanthamoeba. The immune defense mechanisms that operate against Acanthamoeba have not been well characterized, but it has been proposed that both innate and acquired immunity play a role. The ameba's life cycle includes an active feeding trophozoite stage and a dormant cyst stage. Trophozoites feed on bacteria, yeast, and algae. However, both trophozoites and cysts can retain viable bacteria and may serve as reservoirs for bacteria with human pathogenic potential. Diagnosis of infection includes direct microscopy of wet mounts of cerebrospinal fluid or stained smears of cerebrospinal fluid sediment, light or electron microscopy of tissues, in vitro cultivation of Acanthamoeba, and histological assessment of frozen or paraffin-embedded sections of brain or cutaneous lesion biopsy material. Immunocytochemistry, chemifluorescent dye staining, PCR, and analysis of DNA sequence variation also have been employed for laboratory diagnosis. Treatment of Acanthamoeba infections has met with mixed results. However, chlorhexidine gluconate, alone or in combination with propamidene isethionate, is effective in some patients. Furthermore, effective treatment is complicated since patients may present with underlying disease and Acanthamoeba infection may not be recognized. Since an increase in the number of cases of Acanthamoeba infections has occurred worldwide, these protozoa have become increasingly important as agents of human disease.
Figures

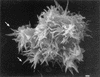
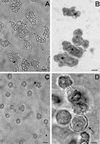

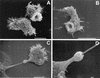

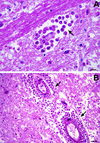



References
-
- Abu Kwaik, Y., L. Y. Gao, B. J. Stone, and O. S. Harb. 1998. Invasion of mammalian and protozoan cells by Legionella pneumophila. Bull. Inst. Pasteur 96:237-247.
-
- Alfieri, S. C., C. E. Correia, S. A. Motegi, and E. M. Pral. 2000. Proteinase activities in total extracts and in medium conditioned by Acanthamoeba polyphaga trophozoites. J. Parasitol. 86:220-227. - PubMed
-
- Alizadeh, H., S. Apte, M. S. El Agha, L. Li, M. Hurt, K. Howard, H. D. Cavanagh, J. P. McCulley, and J. Y. Niederkorn. 2001. Tear IgA and serum IgG antibodies against Acanthamoeba in patients with Acanthamoeba keratitis. Cornea 20:622-627. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

