Synaptically activated Ca2+ waves in layer 2/3 and layer 5 rat neocortical pyramidal neurons
- PMID: 12692172
- PMCID: PMC2342955
- DOI: 10.1113/jphysiol.2002.037614
Synaptically activated Ca2+ waves in layer 2/3 and layer 5 rat neocortical pyramidal neurons
Abstract
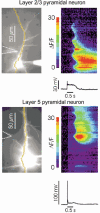
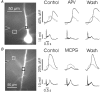
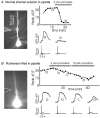
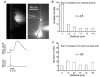
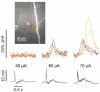
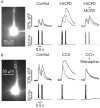
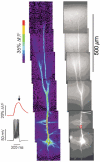
Calcium waves in layer 2/3 and layer 5 neocortical somatosensory pyramidal neurons were examined in slices from 2- to 8-week-old rats. Repetitive synaptic stimulation evoked a delayed, all-or-none [Ca2+]i increase primarily on the main dendritic shaft. This component was blocked by 1 mM (R,S)-alpha-methyl-4-carboxyphenylglycine (MCPG), 10 microM ryanodine, 1 mg ml-1 internal heparin, and was not blocked by 400 microM internal Ruthenium Red, indicating that it was due to Ca2+ release from internal stores by inositol 1,4,5-trisphosphate (IP3) mobilized via activation of metabotropic glutamate receptors. Calcium waves were initiated on the apical shaft at sites between the soma to around the main branch point, mostly at insertion points of oblique dendrites, and spread in both directions along the shaft. In the proximal dendrites the peak amplitude of the resulting [Ca2+]i change was much larger than that evoked by a train of Na+ spikes. In distal dendrites the peak amplitude was comparable to the [Ca2+]i change due to a Ca2+ spike. IP3-mediated Ca2+ release also was observed in the presence of the metabotropic agonists t-ACPD and carbachol when backpropagating spikes were generated. Ca2+ entry through NMDA receptors was observed primarily on the oblique dendrites. The main differences between waves in neocortical neurons and in previously described hippocampal pyramidal neurons were, (a) Ca2+ waves in L5 neurons could be evoked further out along the main shaft, (b) Ca2+ waves extended slightly further out into the oblique dendrites and (c) higher concentrations of bath-applied t-ACPD and carbachol were required to generate Ca2+ release events by backpropagating action potentials.
Figures


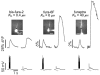


Comment in
-
Synaptically driven Ca2+ release in hippocampal neurons.J Neurophysiol. 2003 Nov;90(5):3579; author reply 3579-80. doi: 10.1152/jn.00731.2003. J Neurophysiol. 2003. PMID: 14615437 No abstract available.
Similar articles
-
Inositol 1,4,5-trisphosphate (IP3)-mediated Ca2+ release evoked by metabotropic agonists and backpropagating action potentials in hippocampal CA1 pyramidal neurons.J Neurosci. 2000 Nov 15;20(22):8365-76. doi: 10.1523/JNEUROSCI.20-22-08365.2000. J Neurosci. 2000. PMID: 11069943 Free PMC article.
-
Modulation of calcium wave propagation in the dendrites and to the soma of rat hippocampal pyramidal neurons.J Physiol. 2006 Sep 1;575(Pt 2):455-68. doi: 10.1113/jphysiol.2006.114231. Epub 2006 Jun 29. J Physiol. 2006. PMID: 16809362 Free PMC article.
-
Spatial segregation and interaction of calcium signalling mechanisms in rat hippocampal CA1 pyramidal neurons.J Physiol. 2002 Sep 1;543(Pt 2):465-80. doi: 10.1113/jphysiol.2002.020362. J Physiol. 2002. PMID: 12205182 Free PMC article.
-
IP3-dependent calcium-induced calcium release mediates bidirectional calcium waves in neurones: functional implications for synaptic plasticity.Biochim Biophys Acta. 2002 Nov 4;1600(1-2):12-8. doi: 10.1016/s1570-9639(02)00439-9. Biochim Biophys Acta. 2002. PMID: 12445454 Review.
-
Synaptically activated ca2+ release from internal stores in CNS neurons.Cell Mol Neurobiol. 2005 Mar;25(2):283-95. doi: 10.1007/s10571-005-3060-0. Cell Mol Neurobiol. 2005. PMID: 16047542 Free PMC article. Review.
Cited by
-
Chemogenetic Activation of Excitatory Neurons Alters Hippocampal Neurotransmission in a Dose-Dependent Manner.eNeuro. 2019 Nov 15;6(6):ENEURO.0124-19.2019. doi: 10.1523/ENEURO.0124-19.2019. Print 2019 Nov/Dec. eNeuro. 2019. PMID: 31645362 Free PMC article.
-
Priming of intracellular calcium stores in rat CA1 pyramidal neurons.J Physiol. 2007 Oct 1;584(Pt 1):75-87. doi: 10.1113/jphysiol.2007.137661. Epub 2007 Aug 9. J Physiol. 2007. PMID: 17690146 Free PMC article.
-
Initiation and propagation of a neuronal intracellular calcium wave.J Comput Neurosci. 2008 Oct;25(2):334-48. doi: 10.1007/s10827-008-0082-x. Epub 2008 Mar 5. J Comput Neurosci. 2008. PMID: 18320300
-
An intracellular Ca2+ subsystem as a biologically plausible source of intrinsic conditional bistability in a network model of working memory.J Comput Neurosci. 2006 Feb;20(1):97-107. doi: 10.1007/s10827-006-4791-8. Epub 2006 Feb 20. J Comput Neurosci. 2006. PMID: 16511655
-
Compartment-specific tuning of dendritic feature selectivity by intracellular Ca2+ release.Science. 2022 Mar 18;375(6586):eabm1670. doi: 10.1126/science.abm1670. Epub 2022 Mar 18. Science. 2022. PMID: 35298275 Free PMC article.
References
-
- Baimbridge KG, Miller JJ, Parkes CO. Calcium-binding protein distribution in the rat brain. Brain Res. 1982;239:519–525. - PubMed
-
- Berridge MJ. Neuronal calcium signaling. Neuron. 1998;21:13–26. - PubMed
-
- Bezprozvanny I, Watras J, Ehrlich BE. Bell-shaped calcium response curves of Ins(1, 4, 5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991;351:751–754. - PubMed
-
- Conn PJ, Pin JP. Pharmacology and function of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. - PubMed
-
- Daw MI, Bortolotto ZA, Saulle E, Zaman S, Collingridge GL, Isaac JT. Phosphatidylinositol 3 kinase regulates synapse specificity of hippocampal long-term depression. Nature Neurosci. 2002;5:835–836. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

