Expression and splicing of the insulin-like growth factor gene in rodent muscle is associated with muscle satellite (stem) cell activation following local tissue damage
- PMID: 12692175
- PMCID: PMC2342958
- DOI: 10.1113/jphysiol.2002.035832
Expression and splicing of the insulin-like growth factor gene in rodent muscle is associated with muscle satellite (stem) cell activation following local tissue damage
Abstract
Muscle satellite cells are mononuclear cells that remain in a quiescent state until activated when they proliferate and fuse with muscle fibres to donate nuclei, a process necessary for post-embryonic growth, hypertrophy and tissue repair in this post-mitotic tissue. These processes have been associated with expression of the insulin-like growth factor (IGF-I) gene that can undergo alternative splicing to generate different gene products with varying functions. To gain insight into the cellular mechanisms involved in local tissue repair, the time courses of expression of two IGF-I splice variants produced in muscle were determined together with marker genes for satellite cell activation following local muscle damage. Using real-time RT-PCR with specific primers, the mRNA transcripts in rat tibialis anterior muscles were measured at different time intervals following either mechanical damage imposed by electrical stimulation of the stretched muscle or damage caused by injection with bupivacaine. It was found that the autocrine splice variant mechano growth factor (MGF) was rapidly expressed and then declined within a few days following both types of damage. Systemic IGF-IEa was more slowly upregulated and its increase was commensurate with the rate of decline in MGF expression. Satellite cell activation as measured by M-cadherin and one of the muscle regulatory factors MyoD and the sequence of expression suggests that the initial pulse of MGF is responsible for satellite cell activation, as the systemic IGF-IEa mRNA expression peaks after the expression of these markers, including M-cadherin protein. Later splicing of the IGF-I gene away from MGF but towards IGF-IEa seems physiologically appropriate as IGF-IEa is the main source of mature IGF-I for upregulation of protein synthesis required to complete the repair.
Figures
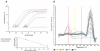
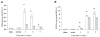
 , right TA, contralateral. For all groups n = 6. Values are expressed as means ±
, right TA, contralateral. For all groups n = 6. Values are expressed as means ±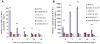
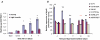
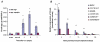

Similar articles
-
Muscle satellite (stem) cell activation during local tissue injury and repair.J Anat. 2003 Jul;203(1):89-99. doi: 10.1046/j.1469-7580.2003.00195.x. J Anat. 2003. PMID: 12892408 Free PMC article.
-
Short-term strength training and the expression of myostatin and IGF-I isoforms in rat muscle and tendon: differential effects of specific contraction types.J Appl Physiol (1985). 2007 Feb;102(2):573-81. doi: 10.1152/japplphysiol.00866.2006. Epub 2006 Oct 12. J Appl Physiol (1985). 2007. PMID: 17038487
-
Growth hormone stimulates mechano growth factor expression and activates myoblast transformation in C2C12 cells.Kobe J Med Sci. 2008 May 23;54(1):E46-54. Kobe J Med Sci. 2008. PMID: 18772608
-
Minireview: Mechano-growth factor: a putative product of IGF-I gene expression involved in tissue repair and regeneration.Endocrinology. 2010 Mar;151(3):865-75. doi: 10.1210/en.2009-1217. Epub 2010 Feb 3. Endocrinology. 2010. PMID: 20130113 Free PMC article. Review.
-
Skeletal muscle hypertrophy and regeneration: interplay between the myogenic regulatory factors (MRFs) and insulin-like growth factors (IGFs) pathways.Cell Mol Life Sci. 2013 Nov;70(21):4117-30. doi: 10.1007/s00018-013-1330-4. Epub 2013 Apr 4. Cell Mol Life Sci. 2013. PMID: 23552962 Free PMC article. Review.
Cited by
-
Stem cell activation in skeletal muscle regeneration.Cell Mol Life Sci. 2015 May;72(9):1663-77. doi: 10.1007/s00018-014-1819-5. Epub 2015 Jan 9. Cell Mol Life Sci. 2015. PMID: 25572293 Free PMC article. Review.
-
Local NSAID infusion inhibits satellite cell proliferation in human skeletal muscle after eccentric exercise.J Appl Physiol (1985). 2009 Nov;107(5):1600-11. doi: 10.1152/japplphysiol.00707.2009. Epub 2009 Aug 27. J Appl Physiol (1985). 2009. PMID: 19713429 Free PMC article. Clinical Trial.
-
Age-related decreases of serum-response factor levels in human mesenchymal stem cells are involved in skeletal muscle differentiation and engraftment capacity.Stem Cells Dev. 2014 Jun 1;23(11):1206-16. doi: 10.1089/scd.2013.0231. Epub 2014 Apr 1. Stem Cells Dev. 2014. PMID: 24576136 Free PMC article.
-
Co-expression of IGF-1 family members with myogenic regulatory factors following acute damaging muscle-lengthening contractions in humans.J Physiol. 2008 Nov 15;586(22):5549-60. doi: 10.1113/jphysiol.2008.160176. Epub 2008 Sep 25. J Physiol. 2008. PMID: 18818249 Free PMC article.
-
Skeletal Muscle Recovery from Disuse Atrophy: Protein Turnover Signaling and Strategies for Accelerating Muscle Regrowth.Int J Mol Sci. 2020 Oct 26;21(21):7940. doi: 10.3390/ijms21217940. Int J Mol Sci. 2020. PMID: 33114683 Free PMC article. Review.
References
-
- Adams GR. Exercise effects on muscle insulin signaling and action. Invited review: autocrine/paracrine IGF-I and skeletal muscle adaptation. J Physiol. 1993;96:1159–1162. - PubMed
-
- Adams GR. Role of insulin-like growth factor-I in the regulation of skeletal muscle adaptation to increased loading. Exerc Sport Sci Rev. 1998;26:31–60. - PubMed
-
- Barton-Davis ER, Shoturma DI, Sweeney HL. Contribution of satellite cells to IGF-I induced hypertrophy of skeletal muscle. Acta Physiol Scand. 1999;167:301–305. - PubMed
-
- Bradley WG. Muscle fibre splitting. In: Mauro A, editor. Muscle Regeneration. New York: Raven Press Ltd; 1979. pp. 215–232.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

