Attenuated Salmonella enterica serovar Typhi and Shigella flexneri 2a strains mucosally deliver DNA vaccines encoding measles virus hemagglutinin, inducing specific immune responses and protection in cotton rats
- PMID: 12692223
- PMCID: PMC153971
- DOI: 10.1128/jvi.77.9.5209-5217.2003
Attenuated Salmonella enterica serovar Typhi and Shigella flexneri 2a strains mucosally deliver DNA vaccines encoding measles virus hemagglutinin, inducing specific immune responses and protection in cotton rats
Abstract
Measles remains a leading cause of child mortality in developing countries. Residual maternal measles antibodies and immunologic immaturity dampen immunogenicity of the current vaccine in young infants. Because cotton rat respiratory tract is susceptible to measles virus (MV) replication after intranasal (i.n.) challenge, this model can be used to assess the efficacy of MV vaccines. Pursuing a new measles vaccine strategy that might be effective in young infants, we used attenuated Salmonella enterica serovar Typhi CVD 908-htrA and Shigella flexneri 2a CVD 1208 vaccines to deliver mucosally to cotton rats eukaryotic expression plasmid pGA3-mH and Sindbis virus-based DNA replicon pMSIN-H encoding MV hemagglutinin (H). The initial i.n. dose-response with bacterial vectors alone identified a well-tolerated dosage (1 x 10(9) to 7 x 10(9) CFU) and a volume (20 micro l) that elicited strong antivector immune responses. Animals immunized i.n. on days 0, 28, and 76 with bacterial vectors carrying DNA plasmids encoding MV H or immunized parenterally with these naked DNA vaccine plasmids developed MV plaque reduction neutralizing antibodies and proliferative responses against MV antigens. In a subsequent experiment of identical design, cotton rats were challenged with wild-type MV 1 month after the third dose of vaccine or placebo. MV titers were significantly reduced in lung tissue of animals immunized with MV DNA vaccines delivered either via bacterial live vectors or parenterally. Since attenuated serovar Typhi and S. flexneri can deliver measles DNA vaccines mucosally in cotton rats, inducing measles immune responses (including neutralizing antibodies) and protection, boosting strategies can now be evaluated in animals primed with MV DNA vaccines.
Figures


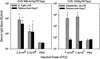
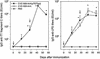
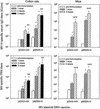
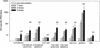
 ), 65 (
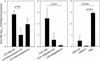
), 65 ( ), and 104 (▪). Responses are expressed as GM titers ± the SE from four to five animals in each group. The numbers of animals that seroconverted, i.e., manifested a fourfold increase in antibody titers, after the third dose within each group are indicated. ✽, Significant increases (P < 0.05) in antibody titers after immunization compared to preimmunization levels and control groups.
), and 104 (▪). Responses are expressed as GM titers ± the SE from four to five animals in each group. The numbers of animals that seroconverted, i.e., manifested a fourfold increase in antibody titers, after the third dose within each group are indicated. ✽, Significant increases (P < 0.05) in antibody titers after immunization compared to preimmunization levels and control groups.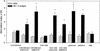
Similar articles
-
Mucosal DNA vaccine immunization against measles with a highly attenuated Shigella flexneri vector.J Immunol. 1999 Feb 1;162(3):1603-10. J Immunol. 1999. PMID: 9973419
-
Sindbis virus-based measles DNA vaccines protect cotton rats against respiratory measles: relevance of antibodies, mucosal and systemic antibody-secreting cells, memory B cells, and Th1-type cytokines as correlates of immunity.J Virol. 2009 Mar;83(6):2789-94. doi: 10.1128/JVI.02191-08. Epub 2009 Jan 7. J Virol. 2009. PMID: 19129445 Free PMC article.
-
Neonatal immunization with a Sindbis virus-DNA measles vaccine induces adult-like neutralizing antibodies and cell-mediated immunity in the presence of maternal antibodies.J Immunol. 2006 May 1;176(9):5671-81. doi: 10.4049/jimmunol.176.9.5671. J Immunol. 2006. PMID: 16622037
-
Animal models paving the way for clinical trials of attenuated Salmonella enterica serovar Typhi live oral vaccines and live vectors.Vaccine. 2003 Jan 17;21(5-6):401-18. doi: 10.1016/s0264-410x(02)00472-3. Vaccine. 2003. PMID: 12531639 Review.
-
Measles: old vaccines, new vaccines.Curr Top Microbiol Immunol. 2009;330:191-212. doi: 10.1007/978-3-540-70617-5_10. Curr Top Microbiol Immunol. 2009. PMID: 19203111 Review.
Cited by
-
Enhanced potency of plasmid DNA microparticle human immunodeficiency virus vaccines in rhesus macaques by using a priming-boosting regimen with recombinant proteins.J Virol. 2005 Jul;79(13):8189-200. doi: 10.1128/JVI.79.13.8189-8200.2005. J Virol. 2005. PMID: 15956564 Free PMC article.
-
Characterization of immune responses induced by intramuscular vaccination with DNA vaccines encoding measles virus hemagglutinin and/or fusion proteins.J Virol. 2005 Aug;79(15):9854-61. doi: 10.1128/JVI.79.15.9854-9861.2005. J Virol. 2005. PMID: 16014946 Free PMC article.
-
A Microneedle Patch for Measles and Rubella Vaccination Is Immunogenic and Protective in Infant Rhesus Macaques.J Infect Dis. 2018 Jun 5;218(1):124-132. doi: 10.1093/infdis/jiy139. J Infect Dis. 2018. PMID: 29701813 Free PMC article.
-
Enteric pathogens as vaccine vectors for foreign antigen delivery.Infect Immun. 2004 Oct;72(10):5535-47. doi: 10.1128/IAI.72.10.5535-5547.2004. Infect Immun. 2004. PMID: 15385450 Free PMC article. Review. No abstract available.
-
Live Bacterial Vectors-A Promising DNA Vaccine Delivery System.Med Sci (Basel). 2018 Mar 23;6(2):27. doi: 10.3390/medsci6020027. Med Sci (Basel). 2018. PMID: 29570602 Free PMC article. Review.
References
-
- Aaby, P., K. Knudsen, T. G. Jensen, J. Tharup, A. Poulsen, M. Sodemann, M. C. da Silva, and H. Whittle. 1990. Measles incidence, vaccine efficacy, and mortality in two urban African areas with high vaccination coverage. J. Infect. Dis. 162:1043-1048. - PubMed
-
- Aaby, P., K. Knudsen, H. Whittle, I. M. Lisse, J. Thaarup, A. Poulsen, M. Sodemann, M. Jakobsen, L. Brink, U. Gansted, A. Permin, T. G. Jensen, H. Andersen, and M. C. da Silva. 1993. Long-term survival after Edmonston-Zagreb measles vaccination in Guinea-Bissau: increased female mortality rate. J. Pediatr. 122:904-908. - PubMed
-
- Albrecht, P., K. Herrmann, and G. R. Burns. 1981. Role of virus strain in conventional and enhanced measles plaque neutralization test. J. Virol. Methods 3:251-260. - PubMed
-
- Altboum, Z., E. M. Barry, G. Losonsky, J. E. Galen, and M. M. Levine. 2001. Attenuated Shigella flexneri 2a ΔguaBA strain CVD 1204 expressing enterotoxigenic Escherichia coli (ETEC) CS2 and CS3 fimbriae as a live mucosal vaccine against Shigella and ETEC infection. Infect. Immun. 69:3150-3158. - PMC - PubMed
-
- Anderson, R., M. F. Pasetti, M. B. Sztein, M. M. Levine, and F. N. Noriega. 2000. Δgua attenuated Shigella flexneri 2a strain CVD 1204 as a Shigella vaccine and as a live mucosal delivery system for fragment C of tetanus toxin. Vaccine 18:2193-2202. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

