A new model of busulphan-induced chronic bone marrow aplasia in the female BALB/c mouse
- PMID: 12694485
- PMCID: PMC2517539
- DOI: 10.1046/j.1365-2613.2003.00239.x
A new model of busulphan-induced chronic bone marrow aplasia in the female BALB/c mouse
Abstract
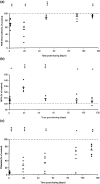
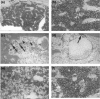
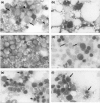
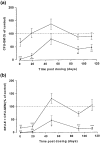
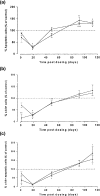
Aplastic anaemia (AA) is characterized by hypocellular marrow, pancytopenia, and risk of severe anaemia, haemorrhage and infection. AA is often idiopathic, but frequently occurs after exposure to drugs/chemicals. However, the pathogenesis of AA is not clearly understood, and there are no convenient animal models of drug-induced AA. We have evaluated regimens of busulphan (BU) administration in the mouse to produce a model of chronic bone marrow aplasia showing features of human AA. Mice were given 8 doses of BU at 0, 5.25 and 10.50 mg/kg over 23 days; marrow and blood samples were examined at 1, 19, 49, 91 and 112 days after dosing. At day 1 post dosing, in mice treated at 10.50 mg/kg, nucleated marrow cells, CFU-GM and Erythroid-CFU were reduced. Similarly, peripheral blood erythrocytes, leucocytes, platelets and reticulocytes were reduced. At day 19 and 49 post dosing, there was a trend for parameters to return towards normal. However, at day 91 and 112 post dosing, values remained significantly depressed, with a stabilized chronic bone marrow aplasia. At day 91 and 112 post dosing, marrow cell counts, CFU-GM and Erythroid-CFU were decreased; marrow nucleated cell apoptosis and c-kit+ cell apoptosis were increased; peripheral blood erythrocyte, leucocyte, and platelet counts were reduced. We conclude that this is a model of chronic bone marrow aplasia which has many interesting features of AA. The model is convenient to use and has potential in several areas, particularly for investigations on mechanisms of AA pathogenesis in man.
Figures

References
-
- Andrews CM, Dash LM, Williams TC, Craig Gray J, Turton JA. Long-term effects of busulphan on lymphocyte subpopulations in female B6C3F1 mice. Comp. Haematol. Int. 1997;7:230–237.
-
- Andrews CM, Spurling NW, Turton JA. Characterisation of busulphan-induced myelotoxicity in B6C3F1 mice using flow cytometry. Comp. Haematol. Int. 1993;3:538–546.
-
- Andrews CM, Williams TC, Turton JA. Long-term haematological alterations in female B6C3F1 mice treated with busulphan. Comp. Haematol. Int. 1998;8:125–138.
-
- Appelbaum FR, Fefer A. The pathogenesis of aplastic anaemia. Sem. Haematol. 1981;18:241–257. - PubMed
-
- Barnes DWH, Mole RH. Aplastic anaemia in sublethally irradiated mice given allogeneic lymph node cells. Br. J. Haematol. 1967;13:482–491. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

