CD40 signaling is impaired in L. major-infected macrophages and is rescued by a p38MAPK activator establishing a host-protective memory T cell response
- PMID: 12695487
- PMCID: PMC2193877
- DOI: 10.1084/jem.20022033
CD40 signaling is impaired in L. major-infected macrophages and is rescued by a p38MAPK activator establishing a host-protective memory T cell response
Abstract
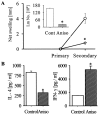
Leishmania, a protozoan parasite, lives and multiplies as amastigote within macrophages. It is proposed that the macrophage expressed CD40 interacts with CD40 ligand on T cells to induce IFN-gamma, a Th1-type cytokine that restricts the amastigote growth. Here, we demonstrate that CD40 cross-linking early after infection resulted in inducible nitric oxide synthetase type-2 (iNOS2) induction and iNOS2-dependent amastigote elimination. Although CD40 expression remained unaltered on L. major-infected macrophages, delay in the treatment of macrophages or of mice with anti-CD40 antibody resulted in significant reduction in iNOS2 expression and leishmanicidal function suggesting impaired CD40 signaling in Leishmania infection. The inhibition of CD40-induced iNOS2 expression by SB203580, a p38-mitogen activated protein kinase (p38MAPK)-specific inhibitor, and the reversal of the inhibition by anisomycin, a p38MAPK activator, suggested a crucial role of p38MAPK in CD40 signaling. Indeed, the CD40-induced p38MAPK phosphorylation, iNOS2 expression and anti-leishmanial function were impaired in Leishmania-infected macrophages but were restored by anisomycin. Anisomycin's effects were reversed by SB203580 emphasizing the role of p38MAPK in CD40-induced iNOS2-dependent leishmanicidal function. Anisomycin administration in L. major-infected BALB/c mice resulted in significant reduction in the parasite load and established a host-protective Th1-type memory response. Also implicated in these findings is a scientific rationale to define novel anti-parasite drug targets and to bypass the problem of drug resistance.
Figures
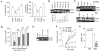

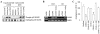
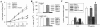
References
-
- Murray, H.W., G.L. Spitalny, and C.F. Nathan. 1985. Activation of mouse peritoneal macrophages in vitro and in vivo by interferon-gamma. J. Immunol. 134:1619–1622. - PubMed
-
- Russell, D.G., and P. Talamas-Rohana. 1989. Leishmania and the macrophage: a marriage of inconvenience. Immunol. Today. 10:328–333. - PubMed
-
- Olivier, M., B.J. Romero-Gallo, C. Matte, J. Blanchette, B.I. Posner, M.J. Tremblay, and R. Faure. 1998. Modulation of interferon-gamma-induced macrophage activation by phosphotyrosine phosphatases inhibition. Effect on murine leishmaniasis progression. J. Biol. Chem. 273:13944–13949. - PubMed
-
- Reiner, S.L., and R.M. Locksley. 1995. The regulation of immunity to Leishmania major. Annu. Rev. Immunol. 13:151–177. - PubMed
-
- Miga, A., S. Master, M. Gonzalez, and R.J. Noelle. 2000. The role of CD40-CD154 interactions in the regulation of cell-mediated immunity. Immunol. Invest. 29:111–114. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

