An important regulatory role for CD4+CD8 alpha alpha T cells in the intestinal epithelial layer in the prevention of inflammatory bowel disease
- PMID: 12695566
- PMCID: PMC154344
- DOI: 10.1073/pnas.0831037100
An important regulatory role for CD4+CD8 alpha alpha T cells in the intestinal epithelial layer in the prevention of inflammatory bowel disease
Abstract
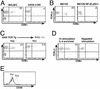
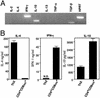
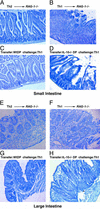
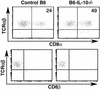
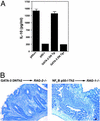
The normal immunoregulatory mechanisms that maintain homeostasis in the intestinal mucosa, despite continuous provocation by environmental antigens, are jeopardized in inflammatory bowel diseases. Although previous studies have suggested that intestinal intraepithelial lymphocytes prevent spontaneous intestinal inflammation, there is limited knowledge about the characteristics of regulatory cells in the intestinal intraepithelial lymphocytes population. Here we show that CD4(+)CD8 alpha alpha(+) double-positive cells present in the intestinal intraepithelial lymphocytes population can suppress T helper 1-induced intestinal inflammation in an IL-10-dependent fashion. CD4(+) T cells stimulated along the Th2 but not the Th1 lineage, when transferred to RAG-1-/- mice, acquire CD8 alpha alpha expression on reaching the intestinal epithelium, and on arrival there, augment their production of IL-10. We show that a precursor CD4(+) T cell after limited, but not repeated, stimulation by IL-4 is able to become a double-positive-regulatory cell on exposure to the intestinal microenvironment in mice. Both CD8 alpha alpha acquisition and IL-10 production depend critically on the NF-kappa B-GATA-3-axis that we have previously shown is essential for differentiation to the Th2 phenotype and for the induction of airway inflammation. Our studies identify a mechanism for the generation of regulatory T cells in the intestine that may play an important role in controlling inflammatory bowel disease.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

