Discrete nuclear structures in actively growing neuroblastoma cells are revealed by antibodies raised against phosphorylated neurofilament proteins
- PMID: 12697053
- PMCID: PMC154097
- DOI: 10.1186/1471-2202-4-6
Discrete nuclear structures in actively growing neuroblastoma cells are revealed by antibodies raised against phosphorylated neurofilament proteins
Abstract
Background: Nuclear objects that have in common the property of being recognized by monoclonal antibodies specific for phosphoprotein epitopes and cytoplasmic intermediate filaments (in particular, SMI-31 and RT-97) have been reported in glial and neuronal cells, in situ and in vitro. Since neurofilament and glial filaments are generally considered to be restricted to the cytoplasm, we were interested in exploring the identity of the structures labeled in the nucleus as well as the conditions under which they could be found there.
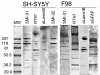
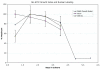
Results: Using confocal microscopy and western analysis techniques, we determined 1) the immunolabeled structures are truly within the nucleus; 2) the phosphoepitope labeled by SMI-31 and RT-97 is not specific to neurofilaments (NFs) and it can be identified on other intermediate filament proteins (IFs) in other cell types; and 3) there is a close relationship between DNA synthesis and the amount of nuclear staining by these antibodies thought to be specific for cytoplasmic proteins. Searches of protein data bases for putative phosphorylation motifs revealed that lamins, NF-H, and GFAP each contain a single tyrosine phosphorylation motif with nearly identical amino acid sequence.
Conclusion: We therefore suggest that this sequence may be the epitope recognized by SMI-31 and RT-97 mABs, and that the nuclear structures previously reported and shown here are likely phosphorylated lamin intermediate filaments, while the cytoplasmic labeling revealed by the same mABs indicates phosphorylated NFs in neurons or GFAP in glia.
Figures


References
-
- Schilling K, Duvernoy C, Keck S, Pilgrim C. Detection and partial characterization of a developmentally regulated nuclear antigen in neural cells in vitro and in vivo. J Histochem Cytochem. 1989;37:241–247. - PubMed
-
- Singh MV, Price KJ, Bhatnagar R, Johnson R, Malhotra SK. J1-31 antigen of astrocytes: cytoplasmic and nuclear localization. Dendron. 1992;1:91–108.
-
- Singh MV, Price KJ, Bhatnagar R, Malhotra SK. Novel rod-shaped structures identified in glioma cell nuclei by immunolabeling and confocal laser fluorescence microscopy. Biomedical Letters. 1994;50:163–172.
-
- Malhotra SK, Bhatnagar R, Shnitka TK, Herrera JJ, Koke JR, Singh MV. Rat glioma cell line as a model for astrogliosis. Cytobios. 1995;82:39–51. - PubMed
-
- Sanford Jeremy. Nuclear Compartments: Nuclear Splicing Speckles. In: Wendy Bickmore, editor. Nuclear Protein Database (NDP) UK, Medical Research Council Human Genetics Unit; 2002. http://npd.hgu.mrc.ac.uk/compartments/speckles.html
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

