Ebola virus infection inversely correlates with the overall expression levels of promyelocytic leukaemia (PML) protein in cultured cells
- PMID: 12697055
- PMCID: PMC154099
- DOI: 10.1186/1471-2180-3-6
Ebola virus infection inversely correlates with the overall expression levels of promyelocytic leukaemia (PML) protein in cultured cells
Abstract
Background: Ebola virus causes severe, often fatal hemorrhagic fever in humans. The mechanism of escape from cellular anti-viral mechanisms is not yet fully understood. The promyelocytic leukaemia (PML) associated nuclear body is part of the interferon inducible cellular defense system. Several RNA viruses have been found to interfere with the anti-viral function of the PML body. The possible interaction between Ebola virus and the PML bodies has not yet been explored.
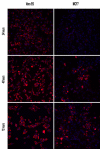
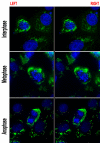
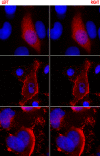
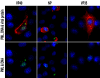
Results: We found that two cell lines, Vero E6 and MCF7, support virus production at high and low levels respectively. The expression of viral proteins was visualized and quantified using high resolution immunofluorescence microscopy. Ebola encoded NP and VP35 accumulated in cytoplasmic inclusion bodies whereas VP40 was mainly membrane associated but it was also present diffusely in the cytoplasm as well as in the euchromatic areas of the nucleus. The anti-VP40 antibody also allowed the detection of extracellular virions. Interferon-alpha treatment decreased the production of all three viral proteins and delayed the development of cytopathic effects in both cell lines. Virus infection and interferon-alpha treatment induced high levels of PML protein expression in MCF7 but much less in Vero E6 cells. No disruption of PML bodies, a common phenomenon induced by a variety of different viruses, was observed.
Conclusion: We have established a simple fixation and immunofluorescence staining procedure that allows specific co-detection and precise sub-cellular localization of the PML nuclear bodies and the Ebola virus encoded proteins NP, VP35 and VP40 in formaldehyde treated cells. Interferon-alpha treatment delays virus production in vitro. Intact PML bodies may play an anti-viral role in Ebola infected cells.
Figures





References
-
- Sanchez A, Khan AS, Zaki SR, Nabel GJ, Ksiazek TG, Peters CJ. Filoviridae: Marburg and Ebola viruses. Fields Virology, DMKnipe and PM Howley, eds Philadelpia: Lippincotts, Williams and Wilkins. 2001. pp. 1279–1304.
-
- Peters CJ, LeDuc JW. An introduction to Ebola: the virus and the disease. J Infect Dis. 1999;179 Suppl 1:ix–xvi. - PubMed
-
- Villinger F, Rollin PE, Brar SS, Chikkala NF, Winter J, Sundstrom JB, Zaki SR, Swanepoel R, Ansari AA, Peters CJ. Markedly elevated levels of interferon (IFN)-gamma, IFN-alpha, interleukin (IL)-2, IL-10, and tumor necrosis factor-alpha associated with fatal Ebola virus infection. J Infect Dis. 1999;179 Suppl 1:S188–NaN. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

