TOX defines a conserved subfamily of HMG-box proteins
- PMID: 12697058
- PMCID: PMC155677
- DOI: 10.1186/1471-2164-4-13
TOX defines a conserved subfamily of HMG-box proteins
Abstract
Background: HMG-box proteins are a large and diverse superfamily of architectural factors that share one or more copies of a sequence- and structurally-related DNA binding domain. These proteins can modify chromatin structure by bending and unwinding DNA. HMG-box proteins can be divided into two subfamilies based on whether they recognize DNA in a sequence-dependent or sequence-independent manner. We recently identified an HMG-box protein involved in T cell development, designated TOX, which is highly conserved in humans and mice.
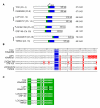
Results: We show here that based on sequence alignment, TOX best fits into the sequence-independent HMG-box family. Three other human and murine predicted proteins are identified that share a common HMG-box domain with TOX, as well as other features. The gene encoding one of these additional family members has a distinct but overlapping pattern of tissue expression when compared to TOX. In addition, we identify genes encoding predicted TOX HMG-box subfamily members in pufferfish and mosquito.
Conclusions: We have identified a novel subfamily of HMG-box proteins that is related to the recently described TOX protein. The highly conserved nature of the TOX family of proteins in humans and mice and differences in the pattern of expression between family members suggest non-overlapping functions of individual proteins. In addition, our data suggest that the TOX subtype of HMG-box domain first appeared in invertebrates, was duplicated in early vertebrates and likely took on new functions in mammalian species.
Figures


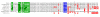
Similar articles
-
The Arabidopsis genome encodes structurally and functionally diverse HMGB-type proteins.J Mol Biol. 2006 May 5;358(3):654-64. doi: 10.1016/j.jmb.2006.02.068. Epub 2006 Mar 10. J Mol Biol. 2006. PMID: 16563436
-
A new mode of DNA binding distinguishes Capicua from other HMG-box factors and explains its mutation patterns in cancer.PLoS Genet. 2017 Mar 9;13(3):e1006622. doi: 10.1371/journal.pgen.1006622. eCollection 2017 Mar. PLoS Genet. 2017. PMID: 28278156 Free PMC article.
-
TOX: an HMG box protein implicated in the regulation of thymocyte selection.Nat Immunol. 2002 Mar;3(3):272-80. doi: 10.1038/ni767. Epub 2002 Feb 19. Nat Immunol. 2002. PMID: 11850626
-
Role, function and regulation of the thymocyte selection-associated high mobility group box protein in CD8+ T cell exhaustion.Immunol Lett. 2021 Jan;229:1-7. doi: 10.1016/j.imlet.2020.11.004. Epub 2020 Nov 11. Immunol Lett. 2021. PMID: 33186634 Review.
-
The HMG-box: a versatile protein domain occurring in a wide variety of DNA-binding proteins.Cell Mol Life Sci. 2007 Oct;64(19-20):2590-606. doi: 10.1007/s00018-007-7162-3. Cell Mol Life Sci. 2007. PMID: 17599239 Free PMC article. Review.
Cited by
-
Super-enhancer-driven TOX2 mediates oncogenesis in Natural Killer/T Cell Lymphoma.Mol Cancer. 2023 Apr 10;22(1):69. doi: 10.1186/s12943-023-01767-1. Mol Cancer. 2023. PMID: 37032358 Free PMC article.
-
Age-related DNA hydroxymethylation is enriched for gene expression and immune system processes in human peripheral blood.Epigenetics. 2020 Mar;15(3):294-306. doi: 10.1080/15592294.2019.1666651. Epub 2019 Sep 26. Epigenetics. 2020. PMID: 31506003 Free PMC article.
-
The TOX subfamily: all-round players in the immune system.Clin Exp Immunol. 2022 Jun 23;208(3):268-280. doi: 10.1093/cei/uxac037. Clin Exp Immunol. 2022. PMID: 35485425 Free PMC article. Review.
-
Structural and functional conservation of fungal MatA and human SRY sex-determining proteins.Nat Commun. 2014 Nov 17;5:5434. doi: 10.1038/ncomms6434. Nat Commun. 2014. PMID: 25399555 Free PMC article.
-
Polygenic susceptibility to breast cancer: current state-of-the-art.Future Oncol. 2009 Jun;5(5):689-701. doi: 10.2217/fon.09.29. Future Oncol. 2009. PMID: 19519208 Free PMC article. Review.
References
-
- Goodwin GH, Johns EW. Isolation and characterisation of two calf-thymus chromatin non-histone proteins with high contents of acidic and basic amino acids. Eur J Biochem. 1973;40:215–219. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

