Role of RANK ligand in mediating increased bone resorption in early postmenopausal women
- PMID: 12697741
- PMCID: PMC152939
- DOI: 10.1172/JCI17215
Role of RANK ligand in mediating increased bone resorption in early postmenopausal women
Abstract
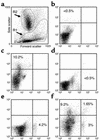
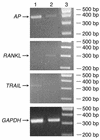
Studies in rodents have implicated various cytokines as paracrine mediators of increased osteoclastogenesis during estrogen deficiency, but increases in RANKL, the final effector of osteoclastogenesis, have not been demonstrated. Thus, we isolated bone marrow mononuclear cells expressing RANKL on their surfaces by two-color flow cytometry using FITC-conjugated osteoprotegerin-Fc (OPG-Fc-FITC) as a probe. The cells were characterized as preosteoblastic marrow stromal cells (MSCs), T lymphocytes, or B lymphocytes by using Ab's against bone alkaline phosphatase (BAP), CD3, and CD20, respectively, in 12 premenopausal women (Group A), 12 early postmenopausal women (Group B), and 12 age-matched, estrogen-treated postmenopausal women (Group C). Fluorescence intensity of OPG-Fc-FITC, an index of the surface concentration of RANKL per cell, was increased in Group B over Groups A and C by two- to threefold for MSCs, T cells, B cells, and total RANKL-expressing cells. Moreover, in the merged groups, RANKL expression per cell correlated directly with the bone resorption markers, serum C-terminal telopeptide of type I collagen and urine N-telopeptide of type I collagen, in all three cell types and inversely with serum 17beta-estradiol for total RANKL-expressing cells. The data suggest that upregulation of RANKL on bone marrow cells is an important determinant of increased bone resorption induced by estrogen deficiency.
Figures

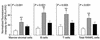
Comment in
-
RANK ligand and the regulation of skeletal remodeling.J Clin Invest. 2003 Apr;111(8):1120-2. doi: 10.1172/JCI18358. J Clin Invest. 2003. PMID: 12697730 Free PMC article. No abstract available.
References
-
- Riggs BL, Khosla S, Melton LJ. Sex steroids and the construction and conservation of the adult skeleton. Endocr. Rev. 2002;23:279–302. - PubMed
-
- Khosla S, Melton LJ, Riggs BL. Estrogen and the male skeleton. J. Clin. Endocrinol. Metab. 2002;87:1443–1450. - PubMed
-
- Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr. Rev. 2000;21:115–137. - PubMed
-
- Pacifici R. Estrogen, cytokines, and pathogenesis of postmenopausal osteoporosis. J. Bone Miner. Res. 1996;11:1043–1051. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

