Immunoregulation of a viral model of multiple sclerosis using the synthetic cannabinoid R+WIN55,212
- PMID: 12697742
- PMCID: PMC152941
- DOI: 10.1172/JCI17652
Immunoregulation of a viral model of multiple sclerosis using the synthetic cannabinoid R+WIN55,212
Abstract
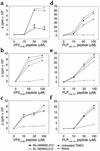
Theiler murine encephalomyelitis virus-induced demyelinating disease (TMEV-IDD) is a mouse model of chronic-progressive multiple sclerosis (MS) characterized by Th1-mediated CNS demyelination and spastic hindlimb paralysis. Existing MS therapies reduce relapse rates in 30% of relapsing-remitting MS patients, but are ineffective in chronic-progressive disease, and their effects on disability progression are unclear. Experimental studies demonstrate cannabinoids are useful for symptomatic treatment of spasticity and tremor in chronic-relapsing experimental autoimmune encephalomyelitis. Cannabinoids, however, have reported immunosuppressive properties. We show that the cannabinoid receptor agonist, R+WIN55,212, ameliorates progression of clinical disease symptoms in mice with preexisting TMEV-IDD. Amelioration of clinical disease is associated with downregulation of both virus and myelin epitope-specific Th1 effector functions (delayed-type hypersensitivity and IFN-gamma production) and the inhibition of CNS mRNA expression coding for the proinflammatory cytokines, TNF-alpha, IL1-beta, and IL-6. Clinical trials investigating the therapeutic potential of cannabinoids for the symptomatic treatment of MS are ongoing, and this study demonstrates that they may also have potent immunoregulatory properties.
Figures
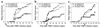

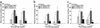
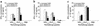
References
-
- Ota K, et al. T-cell recognition of an immunodominant myelin basic protein epitope in multiple sclerosis. Nature. 1990;346:183–187. - PubMed
-
- Goodin DS, et al. Disease modifying therapies in multiple sclerosis – Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and the MS Council for Clinical Practice Guidelines. Neurology. 2002;58:169–178. - PubMed
-
- Devane WA, Dysarz FA, III, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol. Pharmacol. 1988;34:605–613. - PubMed
-
- Munro S, Thomas KL, Abushaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. - PubMed
-
- Galiegue S, et al. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur. J. Biochem. 1995;232:54–61. - PubMed

