Autoinhibition of the kit receptor tyrosine kinase by the cytosolic juxtamembrane region
- PMID: 12697809
- PMCID: PMC153186
- DOI: 10.1128/MCB.23.9.3067-3078.2003
Autoinhibition of the kit receptor tyrosine kinase by the cytosolic juxtamembrane region
Abstract
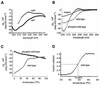
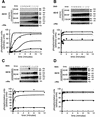
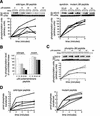
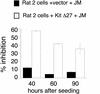
Genetic studies have implicated the cytosolic juxtamembrane region of the Kit receptor tyrosine kinase as an autoinhibitory regulatory domain. Mutations in the juxtamembrane domain are associated with cancers, such as gastrointestinal stromal tumors and mastocytosis, and result in constitutive activation of Kit. Here we elucidate the biochemical mechanism of this regulation. A synthetic peptide encompassing the juxtamembrane region demonstrates cooperative thermal denaturation, suggesting that it folds as an autonomous domain. The juxtamembrane peptide directly interacted with the N-terminal ATP-binding lobe of the kinase domain. A mutation in the juxtamembrane region corresponding to an oncogenic form of Kit or a tyrosine-phosphorylated form of the juxtamembrane peptide disrupted the stability of this domain and its interaction with the N-terminal kinase lobe. Kinetic analysis of the Kit kinase harboring oncogenic mutations in the juxtamembrane region displayed faster activation times than the wild-type kinase. Addition of exogenous wild-type juxtamembrane peptide to active forms of Kit inhibited its kinase activity in trans, whereas the mutant peptide and a phosphorylated form of the wild-type peptide were less effective inhibitors. Lastly, expression of the Kit juxtamembrane peptide in cells which harbor an oncogenic form of Kit inhibited cell growth in a Kit-specific manner. Together, these results show the Kit kinase is autoinhibited through an intramolecular interaction with the juxtamembrane domain, and tyrosine phosphorylation and oncogenic mutations relieved the regulatory function of the juxtamembrane domain.
Figures



References
-
- Barker, S. C., D. B. Kassel, D. Weigl, X. Huang, M. A. Luther, and W. B. Knight. 1995. Characterization of pp60c-src tyrosine kinase activities using a continuous assay: autoactivation of the enzyme is an intermolecular autophosphorylation process. Biochemistry 34:14843-14851. - PubMed
-
- Baxter, R. M., J. P. Secrist, R. R. Vaillancourt, and A. Kazlauskas. 1998. Full activation of the platelet-derived growth factor beta-receptor kinase involves multiple events. J. Biol. Chem. 273:17050-17055. - PubMed
-
- Besmer, P., J. E. Murphy, P. C. George, F. H. Qiu, P. J. Bergold, L. Lederman, H. W. Snyder, Jr., D. Brodeur, E. E. Zuckerman, and W. D. Hardy. 1986. A new acute transforming feline retrovirus and relationship of its oncogene v-kit with the protein kinase gene family. Nature 320:415-421. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
