Histone deacetylase 1/mSin3A disrupts gamma interferon-induced CIITA function and major histocompatibility complex class II enhanceosome formation
- PMID: 12697811
- PMCID: PMC153210
- DOI: 10.1128/MCB.23.9.3091-3102.2003
Histone deacetylase 1/mSin3A disrupts gamma interferon-induced CIITA function and major histocompatibility complex class II enhanceosome formation
Abstract
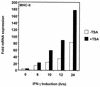
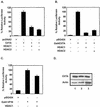
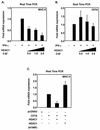
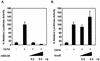
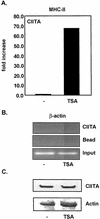
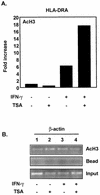
The class II transactivator (CIITA) is a master transcriptional regulator of major histocompatibility complex class II (MHC-II) promoters. CIITA does not bind DNA, but it interacts with the transcription factors RFX5, NF-Y, and CREB and associated chromatin-modifying enzymes to form an enhanceosome. This report examines the effects of histone deacetylases 1 and 2 (HDAC1/HDAC2) on MHC-II gene induction by gamma interferon (IFN-gamma) and CIITA. The results show that an inhibitor of HDACs, trichostatin A, enhances IFN-gamma-induced MHC-II expression, while HDAC1/HDAC2 inhibits IFN-gamma- and CIITA-induced MHC-II gene expression. mSin3A, a corepressor of HDAC1/HDAC2, is important for this inhibition, while NcoR, a corepressor of HDAC3, is not. The effect of this inhibition is directed at CIITA, since HDAC1/HDAC2 reduces transactivation by a GAL4-CIITA fusion protein. CIITA binds to overexpressed and endogenous HDAC1, suggesting that HDAC and CIITA may affect each other by direct or indirect association. Inhibition of HDAC activity dramatically increases the association of NF-YB and RFX5 with CIITA, the assembly of CIITA, NF-YB, and RFX5 enhanceosome, and the extent of H3 acetylation at the MHC-II promoter. These results suggest a model where HDAC1/HDAC2 affect the function of CIITA through a disruption of MHC-II enhanceosome and relevant coactivator-transcription factor association and provide evidence that CIITA may act as a molecular switch to modulate MHC-II transcription by coordinating the functions of both histone acetylases and HDACs.
Figures



Similar articles
-
HDAC2 deacetylates class II transactivator and suppresses its activity in macrophages and smooth muscle cells.J Mol Cell Cardiol. 2009 Mar;46(3):292-9. doi: 10.1016/j.yjmcc.2008.10.023. Epub 2008 Nov 7. J Mol Cell Cardiol. 2009. PMID: 19041327
-
Kaposi's Sarcoma-Associated Herpesvirus Latency-Associated Nuclear Antigen Inhibits Major Histocompatibility Complex Class II Expression by Disrupting Enhanceosome Assembly through Binding with the Regulatory Factor X Complex.J Virol. 2015 May;89(10):5536-56. doi: 10.1128/JVI.03713-14. Epub 2015 Mar 4. J Virol. 2015. PMID: 25740990 Free PMC article.
-
Silencing of transcription of the human luteinizing hormone receptor gene by histone deacetylase-mSin3A complex.J Biol Chem. 2002 Sep 6;277(36):33431-8. doi: 10.1074/jbc.M204417200. Epub 2002 Jun 28. J Biol Chem. 2002. PMID: 12091390
-
The MHC Class II Transactivator CIITA: Not (Quite) the Odd-One-Out Anymore among NLR Proteins.Int J Mol Sci. 2021 Jan 22;22(3):1074. doi: 10.3390/ijms22031074. Int J Mol Sci. 2021. PMID: 33499042 Free PMC article. Review.
-
Structural aspects of the MHC expression control system.Biophys Chem. 2022 May;284:106781. doi: 10.1016/j.bpc.2022.106781. Epub 2022 Feb 15. Biophys Chem. 2022. PMID: 35228036 Free PMC article. Review.
Cited by
-
Interplay among coactivator-associated arginine methyltransferase 1, CBP, and CIITA in IFN-gamma-inducible MHC-II gene expression.Proc Natl Acad Sci U S A. 2005 Nov 8;102(45):16321-6. doi: 10.1073/pnas.0505045102. Epub 2005 Oct 27. Proc Natl Acad Sci U S A. 2005. PMID: 16254053 Free PMC article.
-
Interferon gamma (IFN-γ) disrupts energy expenditure and metabolic homeostasis by suppressing SIRT1 transcription.Nucleic Acids Res. 2012 Feb;40(4):1609-20. doi: 10.1093/nar/gkr984. Epub 2011 Nov 7. Nucleic Acids Res. 2012. PMID: 22064865 Free PMC article.
-
Modulation of antigen-presenting cells by HDAC inhibitors: implications in autoimmunity and cancer.Immunol Cell Biol. 2012 Jan;90(1):55-65. doi: 10.1038/icb.2011.96. Epub 2011 Nov 22. Immunol Cell Biol. 2012. PMID: 22105512 Free PMC article. Review.
-
Expression regulation of major histocompatibility complex class I and class II encoding genes.Front Immunol. 2011 Oct 4;2:48. doi: 10.3389/fimmu.2011.00048. eCollection 2011. Front Immunol. 2011. PMID: 22566838 Free PMC article.
-
MHC class II transactivator represses human IL-4 gene transcription by interruption of promoter binding with CBP/p300, STAT6 and NFAT1 via histone hypoacetylation.Immunology. 2007 Dec;122(4):476-85. doi: 10.1111/j.1365-2567.2007.02674.x. Epub 2007 Jul 20. Immunology. 2007. PMID: 17645498 Free PMC article.
References
-
- Ayer, D. E. 1999. Histone deacetylases: transcriptional repression with SINers and NuRDs. Trends Cell Biol. 9:193-198. - PubMed
-
- Beresford, G. W., and J. M. Boss. 2001. CIITA coordinates multiple histone acetylation modifications at the HLA-DRA promoter. Nat. Immunol. 2:652-657. - PubMed
-
- Cressman, D. E., K. C. Chin, D. J. Taxman, and J. P. Ting. 1999. A defect in the nuclear translocation of CIITA causes a form of type II bare lymphocyte syndrome. Immunity 10:163-171. - PubMed
-
- Cressman, D. E., W. J. O'Connor, S. F. Greer, X. S. Zhu, and J. P. Ting. 2001. Mechanisms of nuclear import and export that control the subcellular localization of class II transactivator. J. Immunol. 167:3626-3634. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
