Putative telomere-recruiting domain in the catalytic subunit of human telomerase
- PMID: 12697823
- PMCID: PMC153184
- DOI: 10.1128/MCB.23.9.3237-3246.2003
Putative telomere-recruiting domain in the catalytic subunit of human telomerase
Abstract
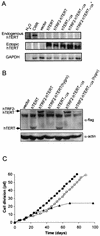
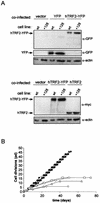
Telomerase, the enzyme that elongates telomeres, is essential to maintain telomere length and to immortalize most cancer cells. However, little is known about the regulation of this enzyme in higher eukaryotes. We previously described a domain in the hTERT telomerase catalytic subunit that is essential for telomere elongation and cell immortalization in vivo but dispensable for catalytic activity in vitro. Here, we show that fusions of hTERT containing different mutations in this domain to the telomere binding protein hTRF2 redirected the mutated hTERT to telomeres and rescued its in vivo functions. We suggest that this domain posttranscriptionally regulates telomerase function by targeting the enzyme to telomeres.
Figures

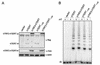
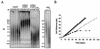
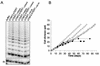
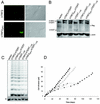
References
-
- Baumann, P., and T. R. Cech. 2001. Pot1, the putative telomere end-binding protein in fission yeast and humans. Science 292:1171-1175. - PubMed
-
- Blackburn, E. H. 2001. Switching and signaling at the telomere. Cell 106:661-673. - PubMed
-
- Broccoli, D., A. Smogorzewska, L. Chong, and T. de Lange. 1997. Human telomeres contain two distinct Myb-related proteins, TRF1 and TRF2. Nat. Genet. 17:231-235. - PubMed
-
- Chong, L., B. van Steensel, D. Broccoli, H. Erdjument-Bromage, J. Hanish, P. Tempst, and T. de Lange. 1995. A human telomeric protein. Science 270:1663-1667. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
