Loss of the Rpb4/Rpb7 subcomplex in a mutant form of the Rpb6 subunit shared by RNA polymerases I, II, and III
- PMID: 12697831
- PMCID: PMC153193
- DOI: 10.1128/MCB.23.9.3329-3338.2003
Loss of the Rpb4/Rpb7 subcomplex in a mutant form of the Rpb6 subunit shared by RNA polymerases I, II, and III
Abstract
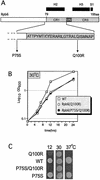
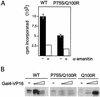
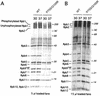
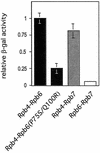
We have identified a conditional mutation in the shared Rpb6 subunit, assembled in RNA polymerases I, II, and III, that illuminated a new role that is independent of its assembly function. RNA polymerase II and III activities were significantly reduced in mutant cells before and after the shift to nonpermissive temperature. In contrast, RNA polymerase I was marginally affected. Although the Rpb6 mutant strain contained two mutations (P75S and Q100R), the majority of growth and transcription defects originated from substitution of an amino acid nearly identical in all eukaryotic counterparts as well as bacterial omega subunits (Q100R). Purification of mutant RNA polymerase II revealed that two subunits, Rpb4 and Rpb7, are selectively lost in mutant cells. Rpb4 and Rpb7 are present at substoichiometric levels, form a dissociable subcomplex, are required for RNA polymerase II activity at high temperatures, and have been implicated in the regulation of enzyme activity. Interaction experiments support a direct association between the Rpb6 and Rpb4 subunits, indicating that Rpb6 is one point of contact between the Rpb4/Rpb7 subcomplex and RNA polymerase II. The association of Rpb4/Rpb7 with Rpb6 suggests that analogous subunits of each RNA polymerase impart class-specific functions through a conserved core subunit.
Figures






References
-
- Asturias, F. J., G. D. Meredith, C. L. Poglitsch, and R. D. Kornberg. 1997. Two conformations of RNA polymerase II revealed by electron crystallography. J. Mol. Biol. 272:536-540. - PubMed
-
- Awrey, D. E., R. G. Weilbaecher, S. A. Hemming, S. M. Orlicky, C. M. Kane, and A. M. Edwards. 1997. Transcription elongation through DNA arrest sites. A multistep process involving both RNA polymerase II subunit RPB9 and TFIIS J. Biol. Chem. 272:14747-14754. - PubMed
-
- Boeke, J. D., J. Trueheart, G. Natsoulis, and G. R. Fink. 1987. 5-Fluoroorotic acid as a selective agent in yeast molecular genetics. Methods Enzymol. 154:164-175. - PubMed
-
- Burke, D., D. Dawson, and T. Stearns. 2000. Methods in yeast genetics. A Cold Spring Harbor Laboratory course manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
