Delivery of bioactive molecules to mitochondria in vivo
- PMID: 12697897
- PMCID: PMC154358
- DOI: 10.1073/pnas.0931245100
Delivery of bioactive molecules to mitochondria in vivo
Abstract
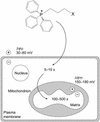
Mitochondrial dysfunction contributes to many human degenerative diseases but specific treatments are hampered by the difficulty of delivering bioactive molecules to mitochondria in vivo. To overcome this problem we developed a strategy to target bioactive molecules to mitochondria by attachment to the lipophilic triphenylphosphonium cation through an alkyl linker. These molecules rapidly permeate lipid bilayers and, because of the large mitochondrial membrane potential (negative inside), accumulate several hundredfold inside isolated mitochondria and within mitochondria in cultured cells. To determine whether this strategy could lead to the development of mitochondria-specific therapies, we investigated the administration and tissue distribution in mice of simple alkyltriphenylphosphonium cations and of mitochondria-targeted antioxidants comprising a triphenylphosphonium cation coupled to a coenzyme Q or vitamin E derivative. Significant doses of these compounds could be fed safely to mice over long periods, coming to steady-state distributions within the heart, brain, liver, and muscle. Therefore, mitochondria-targeted bioactive molecules can be administered orally, leading to their accumulation at potentially therapeutic concentrations in those tissues most affected by mitochondrial dysfunction. This finding opens the way to the testing of mitochondria-specific therapies in mouse models of human degenerative diseases.
Figures




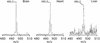
Similar articles
-
Targeting antioxidants to mitochondria by conjugation to lipophilic cations.Annu Rev Pharmacol Toxicol. 2007;47:629-56. doi: 10.1146/annurev.pharmtox.47.120505.105110. Annu Rev Pharmacol Toxicol. 2007. PMID: 17014364 Review.
-
Mitochondrial accumulation of a lipophilic cation conjugated to an ionisable group depends on membrane potential, pH gradient and pK(a): implications for the design of mitochondrial probes and therapies.J Bioenerg Biomembr. 2013 Feb;45(1-2):165-73. doi: 10.1007/s10863-012-9493-5. Epub 2012 Nov 22. J Bioenerg Biomembr. 2013. PMID: 23180142
-
Rapid uptake of lipophilic triphenylphosphonium cations by mitochondria in vivo following intravenous injection: implications for mitochondria-specific therapies and probes.Biochim Biophys Acta. 2010 Sep;1800(9):1009-17. doi: 10.1016/j.bbagen.2010.06.001. Epub 2010 Jun 8. Biochim Biophys Acta. 2010. PMID: 20621583
-
Conjugation of Triphenylphosphonium Cation to Hydrophobic Moieties to Prepare Mitochondria-Targeting Nanocarriers.Methods Mol Biol. 2019;2000:183-189. doi: 10.1007/978-1-4939-9516-5_12. Methods Mol Biol. 2019. PMID: 31148015
-
Molecular strategies for targeting antioxidants to mitochondria: therapeutic implications.Antioxid Redox Signal. 2015 Mar 10;22(8):686-729. doi: 10.1089/ars.2014.5952. Antioxid Redox Signal. 2015. PMID: 25546574 Free PMC article. Review.
Cited by
-
Focus on Mitochondrial Respiratory Chain: Potential Therapeutic Target for Chronic Renal Failure.Int J Mol Sci. 2024 Jan 12;25(2):949. doi: 10.3390/ijms25020949. Int J Mol Sci. 2024. PMID: 38256023 Free PMC article. Review.
-
Pancreatic β Cell Mass Death.Front Pharmacol. 2016 Apr 6;7:83. doi: 10.3389/fphar.2016.00083. eCollection 2016. Front Pharmacol. 2016. PMID: 27092078 Free PMC article. Review.
-
Astragaloside IV Protects Ethanol-Induced Gastric Mucosal Injury by Preventing Mitochondrial Oxidative Stress and the Activation of Mitochondrial Pathway Apoptosis in Rats.Front Pharmacol. 2019 Aug 15;10:894. doi: 10.3389/fphar.2019.00894. eCollection 2019. Front Pharmacol. 2019. PMID: 31474858 Free PMC article.
-
Mitochondrial metabolic dysfunction and non-alcoholic fatty liver disease: new insights from pathogenic mechanisms to clinically targeted therapy.J Transl Med. 2023 Jul 28;21(1):510. doi: 10.1186/s12967-023-04367-1. J Transl Med. 2023. PMID: 37507803 Free PMC article. Review.
-
Dual-targeting pro-apoptotic peptide for programmed cancer cell death via specific mitochondria damage.Sci Rep. 2013 Dec 16;3:3468. doi: 10.1038/srep03468. Sci Rep. 2013. PMID: 24336626 Free PMC article.
References
-
- Puccio H, Simon D, Cossee M, Criqui-Filipe P, Tiziano F, Melki J, Hindelang C, Matyas R, Rustin P, Koenig M. Nat Genet. 2001;27:181–186. - PubMed
-
- Schapira A H, Mann V M, Cooper J M, Krige D, Jenner P J, Marsden C D. Ann Neurol. 1992;32:S116–S124. - PubMed
-
- Nishikawa T, Edelstein D, Du X L, Yamagishi S-I, Matsumura T, Kaneda Y, Yorek M A, Beebe D, Oates P J, Hammes H-P, et al. Nature. 2000;404:787–790. - PubMed
-
- Tabrizi S J, Workman J, Hart P E, Mangiarini L, Mahal A, Bates G, Cooper J M, Schapira A H. Ann Neurol. 2000;47:80–86. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

