Activation of liver X receptor improves glucose tolerance through coordinate regulation of glucose metabolism in liver and adipose tissue
- PMID: 12697904
- PMCID: PMC154360
- DOI: 10.1073/pnas.0830671100
Activation of liver X receptor improves glucose tolerance through coordinate regulation of glucose metabolism in liver and adipose tissue
Abstract
The control of lipid and glucose metabolism is closely linked. The nuclear receptors liver X receptor (LXR)alpha and LXR beta have been implicated in gene expression linked to lipid homeostasis; however, their role in glucose metabolism is not clear. We demonstrate here that the synthetic LXR agonist GW3965 improves glucose tolerance in a murine model of diet-induced obesity and insulin resistance. Analysis of gene expression in LXR agonist-treated mice reveals coordinate regulation of genes involved in glucose metabolism in liver and adipose tissue. In the liver, activation of LXR led to the suppression of the gluconeogenic program including down-regulation of peroxisome proliferator-activated receptor gamma coactivator-1 alpha (PGC-1), phosphoenolpyruvate carboxykinase (PEPCK), and glucose-6-phosphatase expression. Inhibition of gluconeogenic genes was accompanied by an induction in expression of glucokinase, which promotes hepatic glucose utilization. In adipose tissue, activation of LXR led to the transcriptional induction of the insulin-sensitive glucose transporter, GLUT4. We show that the GLUT4 promoter is a direct transcriptional target for the LXR/retinoid X receptor heterodimer and that the ability of LXR ligands to induce GLUT4 expression is abolished in LXR null cells and animals. Consistent with their effects on GLUT4 expression, LXR agonists promote glucose uptake in 3T3-L1 adipocytes in vitro. Thus, activation of LXR alters the expression of genes in liver and adipose tissue that collectively would be expected to limit hepatic glucose output and improve peripheral glucose uptake. These results outline a role for LXRs in the coordination of lipid and glucose metabolism.
Figures

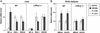



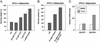

References
-
- Janowski B A, Willy P J, Devi T R, Falck J R, Mangelsdorf D J. Nature. 1996;383:728–731. - PubMed
-
- Lehmann J M, Kliewer S A, Moore L B, Smith-Oliver T A, Oliver B B, Su J L, Sundseth S S, Winegar D A, Blanchard D E, Spencer T A, Willson T M. J Biol Chem. 1997;272:3137–3140. - PubMed
-
- Fu X, Menke J G, Chen Y, Zhou G, MacNaul K L, Wright S D, Sparrow C P, Lund E G. J Biol Chem. 2001;276:38378–38387. - PubMed
-
- Repa J J, Mangelsdorf D J. Annu Rev Cell Dev Biol. 2000;16:459–481. - PubMed
-
- Repa J J, Mangelsdorf D J. Nat Med. 2002;8:1243–1248. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

