Association of stress proteins with autoantigens: a possible mechanism for triggering autoimmunity?
- PMID: 12699405
- PMCID: PMC1808692
- DOI: 10.1046/j.1365-2249.2003.02153.x
Association of stress proteins with autoantigens: a possible mechanism for triggering autoimmunity?
Abstract
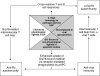
Patterns of autoantibody production are diagnostic of many autoimmune disorders; the recent observation of additional autospecificities towards stress-induced proteins may also provide insight into the mechanisms by which such responses arise. Grp78 (also known as BiP) is a target of autoaggressive B and T cell responses in our murine model of anti-Ro (SS-A) autoimmunity and also in rheumatoid arthritis. In this report we demonstrate reciprocal intermolecular spreading occurs between Ro52 and Grp78 in immunized mice, reflecting physiological association of these molecules in vivo. Moreover, we provide direct biochemical evidence that Grp78 associates with the clinically relevant autoantigen, Ro52 (SS-A). Due to the discrete compartmentalization of Ro52 (nucleocytoplasmic) and Grp78 (endoplasmic reticulum; ER) we propose that association of these molecules occurs either in apoptotic cells, where they have been demonstrated indirectly to co-localize in discrete apoptotic bodies, or in B cells themselves where both Ro52 and Grp78 are known to bind to immunoglobulin heavy chains. Tagging of molecules by association with Grp78 may facilitate receptor mediated phagocytotsis of the complex; we show evidence that exogenous Grp78 can associate with cell surface receptors on a subpopulation of murine splenocytes. Given the likelihood that Grp78 will associate with viral glycoproteins in the ER it is possible that it may become a bystander target of the spreading antiviral immune response. Thus, we propose a model whereby immunity elicited towards Grp78 leads to the selection of responses towards the Ro polypeptides and the subsequent cascade of responses observed in human disease.
Figures

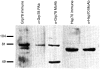

 ) or 287–301 (not shown).
) or 287–301 (not shown).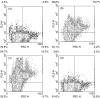
Similar articles
-
Molecular chaperones are targets of autoimmunity in Ro(SS-A) immune mice.Clin Exp Immunol. 1999 Feb;115(2):268-74. doi: 10.1046/j.1365-2249.1999.00794.x. Clin Exp Immunol. 1999. PMID: 9933452 Free PMC article.
-
Spreading of the immune response from 52 kDaRo and 60 kDaRo to calreticulin in experimental autoimmunity.Lupus. 1998;7(1):7-11. doi: 10.1191/096120398678919606. Lupus. 1998. PMID: 9493142
-
The immune response to 52-kDa Ro and 60-kDa Ro is linked in experimental autoimmunity.J Immunol. 1996 Oct 15;157(8):3694-9. J Immunol. 1996. PMID: 8871672
-
Fine specificity of the autoimmune response to the Ro/SSA and La/SSB ribonucleoproteins.Arthritis Rheum. 1999 Feb;42(2):199-209. doi: 10.1002/1529-0131(199902)42:2<199::AID-ANR1>3.0.CO;2-1. Arthritis Rheum. 1999. PMID: 10025913 Review.
-
[Glucose regulated protein 78 kD].Sheng Li Ke Xue Jin Zhan. 2009 Apr;40(2):135-41. Sheng Li Ke Xue Jin Zhan. 2009. PMID: 19558142 Review. Chinese.
Cited by
-
Spreading of antibody reactivity to non-thyroid antigens during experimental immunization with human thyroglobulin.Clin Exp Immunol. 2007 Jan;147(1):120-7. doi: 10.1111/j.1365-2249.2006.03246.x. Clin Exp Immunol. 2007. PMID: 17177971 Free PMC article.
-
Patients with idiopathic pulmonary fibrosis with antibodies to heat shock protein 70 have poor prognoses.Am J Respir Crit Care Med. 2013 Apr 1;187(7):768-75. doi: 10.1164/rccm.201203-0506OC. Am J Respir Crit Care Med. 2013. PMID: 23262513 Free PMC article.
-
Autoantibodies against cell surface GRP78 promote tumor growth in a murine model of melanoma.Melanoma Res. 2011 Feb;21(1):35-43. doi: 10.1097/CMR.0b013e3283426805. Melanoma Res. 2011. PMID: 21164368 Free PMC article.
-
Heat shock proteins: Biological functions, pathological roles, and therapeutic opportunities.MedComm (2020). 2022 Aug 2;3(3):e161. doi: 10.1002/mco2.161. eCollection 2022 Sep. MedComm (2020). 2022. PMID: 35928554 Free PMC article. Review.
-
Cellular stress response and innate immune signaling: integrating pathways in host defense and inflammation.J Leukoc Biol. 2013 Dec;94(6):1167-84. doi: 10.1189/jlb.0313153. Epub 2013 Aug 29. J Leukoc Biol. 2013. PMID: 23990626 Free PMC article. Review.
References
-
- Stephanou A, Latchman DS, Isenberg DA. The regulation of heat shock proteins and their role in systemic lupus erythematosus. Semin Arthritis Rheum. 1998;28:155–62. - PubMed
-
- Ghoreishi M. Heat shock proteins in the pathogenesis of inflammatory skin diseases. J Med Dent Sci. 2000;47:143–50. - PubMed
-
- Blass S, Union A, Raymackers J, et al. The stress protein BiP is overexpressed and is a major B and T cell target in rheumatoid arthritis. Arthritis Rheum. 2001;44:761–71. - PubMed
-
- Corrigall VM, Bodman-Smith MD, Fife MS, et al. The human endoplasmic reticulum molecular chaperone BiP is an autoantigen for rheumatoid arthritis and prevents the induction of experimental arthritis. J Immunol. 2001;166:1492–8. - PubMed
-
- Boehm J, Orth T, Van Nguyen P, Soling HD. Systemic lupus erythematosus is associated with increased auto-antibody titers against calreticulin and grp94, but calreticulin is not the Ro/SS-A antigen. Eur J Clin Invest. 1994;24:248–57. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

