Low concentrations of cytokines produced by allergen-stimulated peripheral blood mononuclear cells have potent effects on nasal polyp-derived fibroblasts
- PMID: 12699413
- PMCID: PMC1808710
- DOI: 10.1046/j.1365-2249.2003.02148.x
Low concentrations of cytokines produced by allergen-stimulated peripheral blood mononuclear cells have potent effects on nasal polyp-derived fibroblasts
Abstract
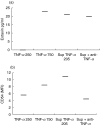
Accumulating data show that fibroblasts are important regulators in the development and maintenance of allergic airway inflammation. However, most studies so far have used individual recombinant cytokines in high concentrations, unlikely to be found in vivo. We aimed to investigate how cytokines produced by peripheral blood mononuclear cells (PBMC) affect fibroblast functions. Primary airway fibroblasts where incubated with allergen-stimulated or non-stimulated PBMC supernatants from allergic patients. The levels of cytokines in PBMC supernatants were measured and the expression of CD54, CD40 and CD106 as well as the production of eotaxin, interleukin (IL)-6 and IL-8 were assessed in fibroblasts. Although the levels of single cytokines measured in PBMC supernatants were low, a significant up-regulation of the surface molecules as well as of IL-6 and IL-8 production was found in fibroblasts cultured with allergen-stimulated PBMC supernatants as compared to non-stimulated, while the increase in eotaxin production was not significant. The evaluation of correlations between cytokines produced by PBMC and effects seen on fibroblasts did not indicate a crucial role for any single cytokine. Furthermore, the addition of comparably low concentrations of recombinant interferon (rIFN)-gamma or recombinant tumour necrosis factor (rTNF)-alpha did not induce the same effects as PBMC supernatants, the only exception being TNF-alpha as a direct inducer of CD54 expression. Our results show that synergistic mechanisms has a more important role than single mediators, highlighting important differences between in vitro experiments, where effects of individual mediators are studied, versus the actual situation in vivo.
Figures

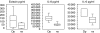


Similar articles
-
Ligation of CD40 on fibroblasts induces CD54 (ICAM-1) and CD106 (VCAM-1) up-regulation and IL-6 production and proliferation.J Leukoc Biol. 1995 Aug;58(2):209-16. doi: 10.1002/jlb.58.2.209. J Leukoc Biol. 1995. PMID: 7543921
-
Fluticasone propionate downregulates nasal fibroblast functions involved in airway inflammation and remodeling.Int Arch Allergy Immunol. 2002 May;128(1):51-8. doi: 10.1159/000058003. Int Arch Allergy Immunol. 2002. PMID: 12037401
-
Inhibitory effect of clarithromycin on costimulatory molecule expression and cytokine production by synovial fibroblast-like cells.Clin Exp Immunol. 1996 Jun;104(3):501-8. doi: 10.1046/j.1365-2249.1996.46752.x. Clin Exp Immunol. 1996. PMID: 9099936 Free PMC article.
-
Interleukin-16 inhibits interleukin-13 production by allergen-stimulated blood mononuclear cells.Immunology. 2006 Jan;117(1):89-96. doi: 10.1111/j.1365-2567.2005.02269.x. Immunology. 2006. PMID: 16423044 Free PMC article.
-
Synergistic induction of thymic stromal lymphopoietin by tumor necrosis factor alpha and Th2 cytokine in nasal polyp fibroblasts.Am J Rhinol Allergy. 2010 Jan-Feb;24(1):e14-8. doi: 10.2500/ajra.2010.24.3436. Am J Rhinol Allergy. 2010. PMID: 20109311
Cited by
-
Dendritic cell-CD4+ T cell interaction: The differential role of IL-4/IL-13 in serum IgE levels in house dust mite allergic patients.Exp Ther Med. 2021 Jan;21(1):95. doi: 10.3892/etm.2020.9527. Epub 2020 Nov 26. Exp Ther Med. 2021. PMID: 33363606 Free PMC article.
-
Human rhinovirus induced cytokine/chemokine responses in human airway epithelial and immune cells.PLoS One. 2014 Dec 12;9(12):e114322. doi: 10.1371/journal.pone.0114322. eCollection 2014. PLoS One. 2014. PMID: 25500821 Free PMC article.
-
Correlation analysis of STAT3 and VEGF expression and eosinophil infiltration in nasal polyps.Eur Arch Otorhinolaryngol. 2015 Aug;272(8):1955-60. doi: 10.1007/s00405-014-3290-1. Epub 2014 Sep 25. Eur Arch Otorhinolaryngol. 2015. PMID: 25253546
-
Role of Fibroblasts in Chronic Inflammatory Signalling in Chronic Rhinosinusitis with Nasal Polyps-A Systematic Review.J Clin Med. 2023 May 4;12(9):3280. doi: 10.3390/jcm12093280. J Clin Med. 2023. PMID: 37176721 Free PMC article. Review.
-
Role played by Th2 type cytokines in IgE mediated allergy and asthma.Lung India. 2010 Apr;27(2):66-71. doi: 10.4103/0970-2113.63609. Lung India. 2010. PMID: 20616938 Free PMC article.
References
-
- Canonica GW, Ciprandi G, Buscaglia S, Pesce G, Bagnasco M. Adhesion molecules of allergic inflammation: recent insights into their functional roles. Allergy. 1994;49:135–41. - PubMed
-
- Passalacqua G, Venturi S, Zoccali P, et al. Cytokine and airways: recent insights and therapeutic implications. Pulmonary Pharmacol Ther. 1998;II:375–9. - PubMed
-
- Brewester CE, Howarth PH, Djukanovic R, Wilson J, Holgate ST, Roche WR. Myofibroblasts and subepithelial fibrosis in bronchial asthma. Am J Respir Cell Mol Biol. 1990;3:507–11. - PubMed
-
- Doucet C, Brouty-Boye D, Pottin-Clemenceau C, Jasmin C, Canonica GW, Azzarone B. IL-4 and IL-13 specifically increase adhesion molecule and inflammatory cytokine expression in human lung fibroblasts. Int Immunol. 1998;10:1421–33. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

