Chaperone-subunit-usher interactions required for donor strand exchange during bacterial pilus assembly
- PMID: 12700251
- PMCID: PMC154394
- DOI: 10.1128/JB.185.9.2723-2730.2003
Chaperone-subunit-usher interactions required for donor strand exchange during bacterial pilus assembly
Abstract
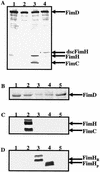
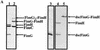
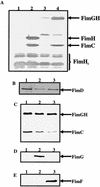
The assembly of type 1 pili on the surface of uropathogenic Escherichia coli proceeds via the chaperone-usher pathway. Chaperone-subunit complexes interact with one another via a process termed donor strand complementation whereby the G1beta strand of the chaperone completes the immunoglobulin (Ig) fold of the pilus subunit. Chaperone-subunit complexes are targeted to the usher, which forms a channel across the outer membrane through which pilus subunits are translocated and assembled into pili via a mechanism known as donor strand exchange. This is a mechanism whereby chaperone uncapping from a subunit is coupled with the simultaneous assembly of the subunit into the pilus fiber. Thus, in the pilus fiber, the N-terminal extension of every subunit completes the Ig fold of its neighboring subunit by occupying the same site previously occupied by the chaperone. Here, we investigated details of the donor strand exchange assembly mechanism. We discovered that the information necessary for targeting the FimC-FimH complex to the usher resides mainly in the FimH protein. This interaction is an initiating event in pilus biogenesis. We discovered that the ability of an incoming subunit (in a chaperone-subunit complex) to participate in donor strand exchange with the growing pilus depended on a previously unrecognized function of the chaperone. Furthermore, the donor strand exchange assembly mechanism between subunits was found to be necessary for subunit translocation across the outer membrane usher.
Figures


References
-
- Amann, E., B. Ochs, and K. J. Abel. 1988. Tightly regulated tac promoter vectors useful for the expression of unfused and fused proteins in Escherichia coli. Gene 69:301-315. - PubMed
-
- Bock, K., M. E. Breimer, A. Brignole, G. C. Hansson, K.-A. Karlsson, G. Larson, H. Leffler, B. E. Samuelsson, N. Strömberg, C. Svanborg-Edén, and J. Thurin. 1985. Specificity of binding of a strain of uropathogenic Escherichia coli to Galα(1-4)Gal-containing glycosphingolipids. J. Biol. Chem. 260:8545-8551. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

