Genetic analysis of pathway specificity during posttranslational protein translocation across the Escherichia coli plasma membrane
- PMID: 12700260
- PMCID: PMC154414
- DOI: 10.1128/JB.185.9.2811-2819.2003
Genetic analysis of pathway specificity during posttranslational protein translocation across the Escherichia coli plasma membrane
Abstract
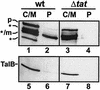
In Escherichia coli, the SecB/SecA branch of the Sec pathway and the twin-arginine translocation (Tat) pathway represent two alternative possibilities for posttranslational translocation of proteins across the cytoplasmic membrane. Maintenance of pathway specificity was analyzed using a model precursor consisting of the mature part of the SecB-dependent maltose-binding protein (MalE) fused to the signal peptide of the Tat-dependent TorA protein. The TorA signal peptide selectively and specifically directed MalE into the Tat pathway. The characterization of a spontaneous TorA signal peptide mutant (TorA*), in which the two arginine residues in the c-region had been replaced by one leucine residue, showed that the TorA*-MalE mutant precursor had acquired the ability for efficiently using the SecB/SecA pathway. Despite the lack of the "Sec avoidance signal," the mutant precursor was still capable of using the Tat pathway, provided that the kinetically favored Sec pathway was blocked. These results show that the h-region of the TorA signal peptide is, in principle, sufficiently hydrophobic for Sec-dependent protein translocation, and therefore, the positively charged amino acid residues in the c-region represent a major determinant for Tat pathway specificity. Tat-dependent export of TorA-MalE was significantly slower in the presence of SecB than in its absence, showing that SecB can bind to this precursor despite the presence of the Sec avoidance signal in the c-region of the TorA signal peptide, strongly suggesting that the function of the Sec avoidance signal is not the prevention of SecB binding; rather, it must be exerted at a later step in the Sec pathway.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

