Isolation of a new hemimethylated DNA binding protein which regulates dnaA gene expression
- PMID: 12700277
- PMCID: PMC154408
- DOI: 10.1128/JB.185.9.2967-2971.2003
Isolation of a new hemimethylated DNA binding protein which regulates dnaA gene expression
Abstract
In this report, we show that yccV, a gene of unknown function, encodes a protein having an affinity for a hemimethylated oriC DNA and that the protein negatively controls dnaA gene expression in vivo.
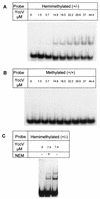
Figures


Similar articles
-
Competition between the replication initiator DnaA and the sequestration factor SeqA for binding to the hemimethylated chromosomal origin of E. coli in vitro.Genes Cells. 2000 Nov;5(11):873-884. doi: 10.1046/j.1365-2443.2000.00380.x. Genes Cells. 2000. PMID: 11122375
-
Genetic organization of the Vibrio harveyi DnaA gene region and analysis of the function of the V. harveyi DnaA protein in Escherichia coli.J Bacteriol. 2002 May;184(9):2533-8. doi: 10.1128/JB.184.9.2533-2538.2002. J Bacteriol. 2002. PMID: 11948168 Free PMC article.
-
The complex of oriC DNA with the DnaA initiator protein.Res Microbiol. 1991 Feb-Apr;142(2-3):119-25. doi: 10.1016/0923-2508(91)90018-6. Res Microbiol. 1991. PMID: 1925008
-
Structure and function of DnaA and the DnaA-box in eubacteria: evolutionary relationships of bacterial replication origins.Mol Microbiol. 1991 Nov;5(11):2589-97. doi: 10.1111/j.1365-2958.1991.tb01967.x. Mol Microbiol. 1991. PMID: 1779750 Review.
-
E. coli minichromosome replication: regulation of initiation at oriC.Res Microbiol. 1991 Feb-Apr;142(2-3):127-30. doi: 10.1016/0923-2508(91)90019-7. Res Microbiol. 1991. PMID: 1925009 Review.
Cited by
-
Deletion of a novel F-box protein, MUS-10, in Neurospora crassa leads to altered mitochondrial morphology, instability of mtDNA and senescence.Genetics. 2010 Aug;185(4):1257-69. doi: 10.1534/genetics.110.117200. Epub 2010 Jun 1. Genetics. 2010. PMID: 20516500 Free PMC article.
-
Coevolution between Nuclear-Encoded DNA Replication, Recombination, and Repair Genes and Plastid Genome Complexity.Genome Biol Evol. 2016 Feb 17;8(3):622-34. doi: 10.1093/gbe/evw033. Genome Biol Evol. 2016. PMID: 26893456 Free PMC article.
-
HspQ Functions as a Unique Specificity-Enhancing Factor for the AAA+ Lon Protease.Mol Cell. 2017 Jun 1;66(5):672-683.e4. doi: 10.1016/j.molcel.2017.05.016. Mol Cell. 2017. PMID: 28575662 Free PMC article.
-
Crystal structure and molecular dynamics of human POLDIP2, a multifaceted adaptor protein in metabolism and genome stability.Protein Sci. 2021 Jun;30(6):1196-1209. doi: 10.1002/pro.4085. Epub 2021 May 10. Protein Sci. 2021. PMID: 33884680 Free PMC article.
-
Molecular characterization and tissue localization of an F-box only protein from silkworm, Bombyx mori.Comp Funct Genomics. 2009;2009:416040. doi: 10.1155/2009/416040. Epub 2009 Jun 14. Comp Funct Genomics. 2009. PMID: 19557136 Free PMC article.
References
-
- Bachmann, B. J. 1987. Linkage map of Escherichia coli K-12, p. 1190-1219. In F. C. Neidhardt, J. L. Ingraham, K. B. Low, B. Magasanik, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella typhimurium: cellular and molecular biology. American Society for Microbiology, Washington, D.C.
-
- Bahloul, A., J. Meury, R. Kern, J. Garwood, S. Guha, and M. Kohiyama. 1996. Co-ordination between membrane oriC sequestration factors and a chromosome partitioning protein, TolC (MukA). Mol. Microbiol. 22:275-282. - PubMed
-
- Blattner, F. R., G. Plunkett III, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1474. - PubMed
-
- Braun, R. E., K. O'Day, and A. Wright. 1985. Autoregulation of the DNA replication gene dnaA in E. coli K-12. Cell 40:159-169. - PubMed
-
- Braun, R. E., and A. Wright. 1986. DNA methylation differentially enhances the expression of one of the two E. coli dnaA promoters in vivo and in vitro. Mol. Gen. Genet. 202:246-250. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

