Human capillary morphogenesis protein 2 functions as an anthrax toxin receptor
- PMID: 12700348
- PMCID: PMC154317
- DOI: 10.1073/pnas.0431098100
Human capillary morphogenesis protein 2 functions as an anthrax toxin receptor
Abstract
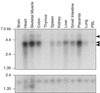
Bacillus anthracis secretes two bipartite toxins thought to be involved in anthrax pathogenesis and resulting death of the host. The current model for intoxication is that protective antigen (PA) toxin subunits bind a single group of cell-surface anthrax toxin receptors (ATRs), encoded by the tumor endothelial marker 8 (TEM8) gene. The ATR/TEM8-PA interaction is mediated by the receptor's extracellular domain related to von Willebrand factor type A or integrin inserted domains (VWA/I domains). A metal ion-dependent adhesion site (MIDAS) located within this domain of the ATR/TEM8 protein chelates a divalent cation critical for PA binding. In this report, we identify a second PA receptor encoded by capillary morphogenesis gene 2 (CMG2), which has 60% amino acid identity to ATR/TEM8 within the VWA/I domain, as well as a conserved MIDAS motif. A recombinant CMG2 protein bound PA and mediated toxin internalization when expressed on receptor-deficient cells. Binding between the CMG2 VWA/I domain and PA was shown to be direct and metal-dependent, although the cation specificity of this interaction is different than that observed with ATR/TEM8. Northern blot analysis revealed that CMG2 is widely expressed in human tissues, indicating that this receptor is likely to be relevant for disease pathogenesis. Finally, a soluble version of the CMG2 VWA/I domain inhibited intoxication of cells expressing endogenous toxin receptors when it was added to PA at a 3:1 ratio. These studies distinguish CMG2 as a second anthrax toxin receptor and identify a potent antitoxin that may prove useful for the treatment of anthrax.
Figures

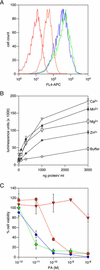
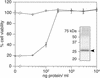
 ), were incubated with 10−9
M PA and with 10−10 M LFN-DTA in the presence
of increasing amounts of CMG2VWA/I-MycHis protein (0–10
μg/ml). Cell viability after intoxication was then measured (as in
Fig. 2) and is shown as a percentage of that obtained with cells
treated with PA alone (100% viable). For control purposes, the same
experiments were performed with PA receptor-deficient CHO-R1.1 cells
(□). (Inset) Coomassie blue-stained
gel of the purified CMG2VWA/I-MycHis protein preparation
(indicated by an arrowhead).
), were incubated with 10−9
M PA and with 10−10 M LFN-DTA in the presence
of increasing amounts of CMG2VWA/I-MycHis protein (0–10
μg/ml). Cell viability after intoxication was then measured (as in
Fig. 2) and is shown as a percentage of that obtained with cells
treated with PA alone (100% viable). For control purposes, the same
experiments were performed with PA receptor-deficient CHO-R1.1 cells
(□). (Inset) Coomassie blue-stained
gel of the purified CMG2VWA/I-MycHis protein preparation
(indicated by an arrowhead).References
-
- Inglesby T V, O'Toole T, Henderson D A, Bartlett J G, Ascher M S, Eitzen E, Friedlander A M, Gerberding J, Hauer J, Hughes J, et al. J Am Med Assoc. 2002;287:2236–2252. - PubMed
-
- Mock M, Fouet A. Annu Rev Microbiol. 2001;55:647–671. - PubMed
-
- Beauregard K E, Collier R J, Swanson J A. Cell Microbiol. 2000;2:251–258. - PubMed
-
- Mogridge J, Cunningham K, Collier R J. Biochemistry. 2002;41:1079–1082. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

