Functional role of L-type Cav1.3 Ca2+ channels in cardiac pacemaker activity
- PMID: 12700358
- PMCID: PMC154381
- DOI: 10.1073/pnas.0935295100
Functional role of L-type Cav1.3 Ca2+ channels in cardiac pacemaker activity
Abstract
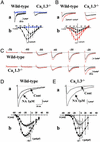
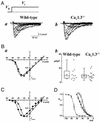
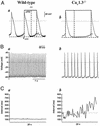
The spontaneous activity of pacemaker cells in the sino-atrial node (SAN) controls the heart rhythm and rate under physiological conditions. Pacemaker activity in SAN cells is due to the presence of the diastolic depolarization, a slow depolarization phase that drives the membrane voltage from the end of an action potential to the threshold of a new action potential. SAN cells express a wide array of ionic channels, but we have limited knowledge about their functional role in pacemaker activity and we still do not know which channels play a prominent role in the generation of the diastolic depolarization. It is thus important to provide genetic evidence linking the activity of genes coding for ionic channels to specific alterations of pacemaker activity of SAN cells. Here, we show that target inactivation of the gene coding for alpha(1D) (Ca(v)1.3) Ca(2+) channels in the mouse not only significantly slows pacemaker activity but also promotes spontaneous arrhythmia in SAN pacemaker cells. These alterations of pacemaker activity are linked to abolition of the major component of the L-type current (I(Ca,L)) activating at negative voltages. Pharmacological analysis of I(Ca,L) demonstrates that Ca(v)1.3 gene inactivation specifically abolishes I(Ca,L) in the voltage range corresponding to the diastolic depolarization. Taken together, our data demonstrate that Ca(v)1.3 channels play a major role in the generation of cardiac pacemaker activity by contributing to diastolic depolarization in SAN pacemaker cells.
Figures


References
-
- Weidmann S. In: The Slow Inward Current and Cardiac Arrhythmias. Zipes D P, Bailey J C, Elharrar V, editors. The Hague, The Netherlands: Nijoff; 1980. pp. 3–9.
-
- DiFrancesco D. Annu Rev Physiol. 1993;55:455–472. - PubMed
-
- Noma A. Jpn Heart J. 1996;37:673–682. - PubMed
-
- Mangoni M E, Nargeot J. Cardiovasc Res. 2001;52:51–64. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

