Functional activity of antibodies against the recombinant OpaJ protein from Neisseria meningitidis
- PMID: 12704102
- PMCID: PMC153225
- DOI: 10.1128/IAI.71.5.2331-2340.2003
Functional activity of antibodies against the recombinant OpaJ protein from Neisseria meningitidis
Abstract
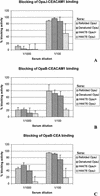
The opacity proteins belong to the major outer membrane proteins of the pathogenic Neisseria and are involved in adhesion and invasion. We studied the functional activity of antibodies raised against the OpaJ protein from strain H44/76. Recombinant OpaJ protein was obtained from Escherichia coli in two different ways: cytoplasmic expression in the form of inclusion bodies followed by purification and refolding and cell surface expression followed by isolation of outer membrane complexes (OMCs). Immunization with purified protein and Quillaja saponin A (QuilA) induced high levels of Opa-specific antibodies, whereas the E. coli OMC preparations generally induced lower levels of antibodies. Two chimeric Opa proteins, hybrids between OpaB and OpaJ, were generated to demonstrate that the hypervariable region 2 is immunodominant. Denatured OpaJ with QuilA induced high levels of immunoglobulin G2a (IgG2a) in addition to IgG1, whereas refolded OpaJ with QuilA induced IgG1 exclusively. These sera did not induce significant complement-mediated killing. However, all sera blocked the interaction of OpaJ-expressing bacteria to CEACAM1-transfected cells. In addition, cross-reactive blocking of OpaB-expressing bacteria to both CEACAM1- and CEA-transfected cells was found for all sera. Sera raised against purified OpaJ and against OpaJ-containing meningococcal OMCs also blocked the nonopsonic interaction of Opa-expressing meningococci with human polymorphonuclear leukocytes.
Figures



References
-
- Abdillahi, H., and J. T. Poolman. 1988. Typing of group B Neisseria meningitidis with monoclonal antibodies in the whole-cell ELISA. J. Med. Microbiol. 26:177-180. - PubMed
-
- Agterberg, M., H. Adriaanse, and J. Tommassen. 1987. Use of outer membrane protein PhoE as a carrier for the transport of a foreign antigenic determinant to the cell surface of Escherichia coli K-12. Gene 59:145-150. - PubMed
-
- Bjune, G., E. A. Hoiby, J. K. Gronnesby, O. Arnesen, J. H. Fredriksen, A. Halstensen, E. Holten, A. K. Lindbak, H. Nokleby, E. Rosenqvist, et al. 1991. Effect of outer membrane vesicle vaccine against group B meningococcal disease in Norway. Lancet 338:1093-1096. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

