Oral immunization with a recombinant malaria protein induces conformational antibodies and protects mice against lethal malaria
- PMID: 12704105
- PMCID: PMC153237
- DOI: 10.1128/IAI.71.5.2356-2364.2003
Oral immunization with a recombinant malaria protein induces conformational antibodies and protects mice against lethal malaria
Abstract
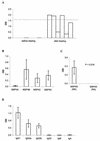
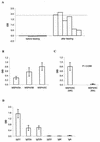
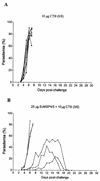
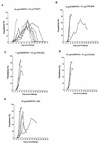
The increasing death toll from malaria, due to the decreasing effectiveness of current prophylactic and therapeutic regimens, has sparked a search for alternative methods of control, such as vaccines. Although several single proteins have shown some promise as subunit vaccines against sexual blood stages in experimental systems, it is clear that multicomponent vaccines are required. Many logistic difficulties make such an approach prohibitively expensive. In an effort to try to overcome some of these issues, we examined the possibility of oral immunization as a route for inducing host protective immunity. We report here that oral feeding of a malaria protein induced serum antibody levels similar to those induced by intraperitoneal immunization with Freund's adjuvant. Further, responses to conformational epitopes were induced. In the rodent challenge system, significant levels of protection to lethal challenge with malaria were induced in mice. The protective efficacy was highly correlated with antibody levels, which depended on the antigen dosage and required cholera toxin subunit B as an oral adjuvant. These findings offer new approaches to the development of a malaria vaccine and provide justification for the investigation of transgenic plants as a means of vaccine delivery.
Figures
Similar articles
-
Immunization with recombinant Plasmodium yoelii merozoite surface protein 4/5 protects mice against lethal challenge.Infect Immun. 2000 Oct;68(10):6034-7. doi: 10.1128/IAI.68.10.6034-6037.2000. Infect Immun. 2000. PMID: 10992516 Free PMC article.
-
Induction of protective polyclonal antibodies by immunization with a Plasmodium yoelii circumsporozoite protein multiple antigen peptide vaccine.J Immunol. 1995 Mar 15;154(6):2784-93. J Immunol. 1995. PMID: 7876549
-
CD4(+) T-cell- and gamma interferon-dependent protection against murine malaria by immunization with linear synthetic peptides from a Plasmodium yoelii 17-kilodalton hepatocyte erythrocyte protein.Infect Immun. 1999 Nov;67(11):5604-14. doi: 10.1128/IAI.67.11.5604-5614.1999. Infect Immun. 1999. PMID: 10531206 Free PMC article.
-
Immunity to erythrocytic stages of malarial parasites.Am J Trop Med Hyg. 1994;50(4 Suppl):27-32. doi: 10.4269/ajtmh.1994.50.27. Am J Trop Med Hyg. 1994. PMID: 7909653 Review.
-
The Case for Exploiting Cross-Species Epitopes in Malaria Vaccine Design.Front Immunol. 2020 Feb 27;11:335. doi: 10.3389/fimmu.2020.00335. eCollection 2020. Front Immunol. 2020. PMID: 32174924 Free PMC article. Review.
Cited by
-
Chloroplast-derived vaccine antigens confer dual immunity against cholera and malaria by oral or injectable delivery.Plant Biotechnol J. 2010 Feb;8(2):223-42. doi: 10.1111/j.1467-7652.2009.00479.x. Epub 2009 Dec 28. Plant Biotechnol J. 2010. PMID: 20051036 Free PMC article.
-
Heteropentameric cholera toxin B subunit chimeric molecules genetically fused to a vaccine antigen induce systemic and mucosal immune responses: a potential new strategy to target recombinant vaccine antigens to mucosal immune systems.Infect Immun. 2005 Sep;73(9):5654-65. doi: 10.1128/IAI.73.9.5654-5665.2005. Infect Immun. 2005. PMID: 16113283 Free PMC article.
-
Bayesian analysis of new and old malaria parasite DNA sequence data demonstrates the need for more phylogenetic signal to clarify the descent of Plasmodium falciparum.Parasitol Res. 2007 Aug;101(3):493-503. doi: 10.1007/s00436-007-0499-6. Epub 2007 Mar 29. Parasitol Res. 2007. PMID: 17393186
-
Growth-inhibitory antibodies are not necessary for protective immunity to malaria infection.Infect Immun. 2010 Feb;78(2):680-7. doi: 10.1128/IAI.00939-09. Epub 2009 Nov 16. Infect Immun. 2010. PMID: 19917716 Free PMC article.
-
Overview of plant-made vaccine antigens against malaria.J Biomed Biotechnol. 2012;2012:206918. doi: 10.1155/2012/206918. Epub 2012 Jul 15. J Biomed Biotechnol. 2012. PMID: 22911156 Free PMC article. Review.
References
-
- Black, C. G., T. Wu, L. Wang, A. R. Hibbs, and R. L. Coppel. 2001. Merozoite surface protein 8 of Plasmodium falciparum contains two epidermal growth factor-like domains. Mol. Biochem. Parasitol. 114:217-226. - PubMed
-
- Camus, D., and T. J. Hadley. 1985. A Plasmodium falciparum antigen that binds to host erythrocytes and merozoites. Science 230:553-556. - PubMed
-
- Galen, J. E., O. G. Gomez-Duarte, G. A. Losonsky, J. L. Halpern, C. S. Lauderbaugh, S. Kaintuck, M. K. Reymann, and M. M. Levine. 1997. A murine model of intranasal immunization to assess the immunogenicity of attenuated Salmonella typhi live vector vaccines in stimulating serum antibody responses to expressed foreign antigens. Vaccine 15:700-708. - PubMed
-
- Ghosh, S., P. Malhotra, P. V. Lalitha, S. Guha-Mukherjee, and V. S. Chauhan. 2002. Expression of Plasmodium falciparum C-terminal region of merozoite surface protein (PfMSP119), a potential malaria vaccine candidate, in tobacco. Plant Sci. 162:335-343.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

