Immunosuppression in hamsters with progressive visceral leishmaniasis is associated with an impairment of protein kinase C activity in their lymphocytes that can be partially reversed by okadaic acid or anti-transforming growth factor beta antibody
- PMID: 12704114
- PMCID: PMC153259
- DOI: 10.1128/IAI.71.5.2439-2446.2003
Immunosuppression in hamsters with progressive visceral leishmaniasis is associated with an impairment of protein kinase C activity in their lymphocytes that can be partially reversed by okadaic acid or anti-transforming growth factor beta antibody
Abstract
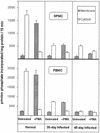
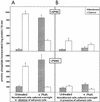
Progressive visceral infection of golden hamsters by Leishmania donovani amastigotes led to gradual impairment of the proliferative responses of their splenic or peripheral blood mononuclear cells (SPMC or PBMC, respectively) to in vitro stimulation with phorbol 12-myristate 13-acetate (PMA) and ionomycin (Io). Removal of macrophage-like adherent cells from SPMC or PBMC of infected animals (I-SPMC or I-PBMC) was earlier shown to restore almost completely their lymphoproliferative responses to PMA plus Io. The present study was directed to evaluate the status of protein kinase C (PKC), a molecule(s) known to play a key role in the lymphoproliferative process. Our results demonstrate that PKC activities (Ca(2+), phosphatidyl serine, and diacyl glycerol dependent) in the cytosolic fraction of untreated nonadherent I-SPMC or I-PBMC as well as in the membrane fraction of PMA-treated cells were decreased significantly relative to those for normal controls. However, removal of adherent cells from I-SPMC or I-PBMC and subsequent overnight in vitro cultivation of nonadherent cells (lymphocytes) resulted in significant restoration of PKC activity in the cytosolic or membrane fraction of untreated or PMA-treated cells, respectively. Partial, though significant, restoration of PKC activity could also be achieved in the membrane fraction of PMA-treated cells following overnight in vitro treatment of I-SPMC or I-PBMC with the Ser/Thr phosphatase inhibitor okadaic acid (OA) or an anti-transforming growth factor beta (anti-TGF-beta) neutralizing antibody. These results correlated well with the ability of OA or the anti-TGF-beta antibody to restore the lymphoproliferative response of I-SPMC or I-PBMC following stimulation with PMA plus Io. Interestingly enough, immunoblotting experiments failed to show any reduction in the level or translocation (following PMA treatment) of conventional PKC isoforms in the SPMC or PBMC of infected animals compared to those of normal controls. The results presented in this study suggest that the adherent cells generated in the SPMC or PBMC of infected animals exert a suppressive effect on the proliferative response of nonadherent cells (lymphocytes) which is likely to be mediated through the downregulation of the activation pathway involving PKC and its downstream molecules such as mitogen-activated protein kinases. Further, the observed suppression of PKC activity and subsequent lymphoproliferative responses can be attributed to alternations in the intracellular phosphorylation-dephosphorylation events. The relevance of these results is discussed in relation to the role of TGF-beta, levels of which are known to be elevated in visceral leishmaniasis.
Figures





Similar articles
-
Immunosuppression in hamsters with progressive visceral leishmaniasis: an evaluation of the role of nitric oxide toward impairment of the lymphoproliferative response.Parasitol Res. 1999 Jul;85(7):594-6. doi: 10.1007/s004360050600. Parasitol Res. 1999. PMID: 10382610
-
Lymph node cells from BALB/c mice with chronic visceral leishmaniasis exhibiting cellular anergy and apoptosis: involvement of Ser/Thr phosphatase.Apoptosis. 2006 Nov;11(11):2013-29. doi: 10.1007/s10495-006-0088-7. Apoptosis. 2006. PMID: 17013755
-
Protein kinase C-dependent and -independent pathways of mitogen-activated protein kinase activation in macrophages by stimuli that activate phospholipase A2.J Biol Chem. 1994 Jul 29;269(30):19480-7. J Biol Chem. 1994. PMID: 8034717
-
Protein kinase C promotes apoptosis in LNCaP prostate cancer cells through activation of p38 MAPK and inhibition of the Akt survival pathway.J Biol Chem. 2003 Sep 5;278(36):33753-62. doi: 10.1074/jbc.M303313200. Epub 2003 Jun 24. J Biol Chem. 2003. PMID: 12824193
-
TGF-beta plays dual roles in immunity and pathogenesis in leishmaniasis.Cytokine. 2025 Mar;187:156865. doi: 10.1016/j.cyto.2025.156865. Epub 2025 Jan 27. Cytokine. 2025. PMID: 39874938 Review.
Cited by
-
A new model of progressive visceral leishmaniasis in hamsters by natural transmission via bites of vector sand flies.J Infect Dis. 2013 Apr 15;207(8):1328-38. doi: 10.1093/infdis/jis932. Epub 2013 Jan 3. J Infect Dis. 2013. PMID: 23288926 Free PMC article.
-
Function of Macrophage and Parasite Phosphatases in Leishmaniasis.Front Immunol. 2017 Dec 22;8:1838. doi: 10.3389/fimmu.2017.01838. eCollection 2017. Front Immunol. 2017. PMID: 29312331 Free PMC article. Review.
-
Role of Cytokines in Experimental and Human Visceral Leishmaniasis.Front Cell Infect Microbiol. 2021 Feb 18;11:624009. doi: 10.3389/fcimb.2021.624009. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33680991 Free PMC article. Review.
-
Sodium antimony gluconate induces generation of reactive oxygen species and nitric oxide via phosphoinositide 3-kinase and mitogen-activated protein kinase activation in Leishmania donovani-infected macrophages.Antimicrob Agents Chemother. 2006 May;50(5):1788-97. doi: 10.1128/AAC.50.5.1788-1797.2006. Antimicrob Agents Chemother. 2006. PMID: 16641451 Free PMC article.
-
Therapy with radio-attenuated vaccine in experimental murine visceral leishmaniasis showed enhanced T cell and inducible nitric oxide synthase levels, suppressed tumor growth factor-beta production with higher expression of some signaling molecules.Braz J Infect Dis. 2015 Jan-Feb;19(1):36-42. doi: 10.1016/j.bjid.2014.10.009. Epub 2014 Dec 19. Braz J Infect Dis. 2015. PMID: 25532783 Free PMC article.
References
-
- Altman, A., K. M. Coggeshall, and T. Mustelin. 1990. Molecular events mediating T cell activation. Adv. Immunol. 48:227-360. - PubMed
-
- Bogdan, C., and M. Rollinghof. 1998. The immune response to Leishmania: mechanism of parasite control and evasion. Int. J. Parasitol. 28:121-134. - PubMed
-
- Bogdan, C., and M. Rollinghof. 1999. How do protozoan parasites survive inside macrophages? Parasitol. Today 15:22-28. - PubMed
-
- Bollag, D. M., M. D. Rozycki, and S. J. Edelstein. 1996. Protein methods, 2nd ed. Wiley-Liss, New York, N.Y.
-
- Bright, J. J., and S. Sriram. 1998. TGF-β inhibits IL-12-induced activation of Jak-STAT pathway in T lymphocytes. J. Immunol. 161:1772-1777. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

