Gamma interferon production by hepatic NK T cells during Escherichia coli infection is resistant to the inhibitory effects of oxidative stress
- PMID: 12704118
- PMCID: PMC153215
- DOI: 10.1128/IAI.71.5.2468-2477.2003
Gamma interferon production by hepatic NK T cells during Escherichia coli infection is resistant to the inhibitory effects of oxidative stress
Abstract
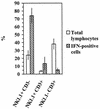
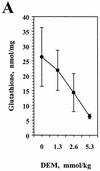
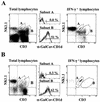
The reductive-oxidative status of tissues regulates the expression of many inflammatory genes that are induced during gram-negative bacterial infections. The cytokine gamma interferon (IFN-gamma) is a potent stimulus for host inflammatory gene expression, and oxidative stress has been shown to inhibit its production in mice challenged with Escherichia coli bacteria. The objective of the present study was to characterize the cells that produced IFN-gamma in a mouse bacterial peritonitis model and determine the effects of oxidative stress on their activation. The liver contained large numbers of IFN-gamma-expressing lymphocytes following challenge with viable E. coli bacteria. The surface phenotypes of IFN-gamma-expressing hepatic lymphocytes were those of natural killer (NK) cells (NK1.1(+) CD3(-)), conventional T cells (NK1.1(-) CD3(+)), and NK T cells (NK1.1(+) CD3(+)). Treating mice with diethyl maleate to deplete tissue thiols significantly impaired IFN-gamma production by NK cells, conventional T cells, and CD1d-restricted NK T cells in response to E. coli challenge. However, IFN-gamma expression by a subset of NK T cells, which did not bind alpha-galactosylceramide-CD1d tetramers, was resistant to the inhibitory effects of tissue oxidative stress. Stress-resistant IFN-gamma-expressing cells were also predominantly CD8(+) and bore gamma delta T-cell antigen receptors. The residual IFN-gamma response by NK T cells may explain previous reports of hepatic gene expression following gram-negative bacterial challenge in thiol-depleted mice. The finding also demonstrates that innate immune cells differ significantly in their responses to altered tissue redox status.
Figures






Similar articles
-
Contribution of NK, NK T, gamma delta T, and alpha beta T cells to the gamma interferon response required for liver protection against Trypanosoma cruzi.Infect Immun. 2006 Apr;74(4):2031-42. doi: 10.1128/IAI.74.4.2031-2042.2006. Infect Immun. 2006. PMID: 16552032 Free PMC article.
-
Gamma interferon prevents the inhibitory effects of oxidative stress on host responses to Escherichia coli infection.Infect Immun. 2001 Apr;69(4):2621-9. doi: 10.1128/IAI.69.4.2621-2629.2001. Infect Immun. 2001. PMID: 11254627 Free PMC article.
-
Activated natural killer T cells producing interferon-gamma elicit promoting activity to murine dendritic cell-based autoimmune hepatic inflammation.Clin Exp Immunol. 2012 Dec;170(3):274-82. doi: 10.1111/j.1365-2249.2012.04664.x. Clin Exp Immunol. 2012. PMID: 23121668 Free PMC article.
-
Requirement for natural killer cell-produced interferon gamma in defense against murine cytomegalovirus infection and enhancement of this defense pathway by interleukin 12 administration.J Exp Med. 1995 Oct 1;182(4):1045-56. doi: 10.1084/jem.182.4.1045. J Exp Med. 1995. PMID: 7561678 Free PMC article.
-
The liver as a crucial organ in the first line of host defense: the roles of Kupffer cells, natural killer (NK) cells and NK1.1 Ag+ T cells in T helper 1 immune responses.Immunol Rev. 2000 Apr;174:35-46. doi: 10.1034/j.1600-0528.2002.017404.x. Immunol Rev. 2000. PMID: 10807505 Review.
Cited by
-
An Oral Salmonella-Based Vaccine Inhibits Liver Metastases by Promoting Tumor-Specific T-Cell-Mediated Immunity in Celiac and Portal Lymph Nodes: A Preclinical Study.Front Immunol. 2016 Mar 1;7:72. doi: 10.3389/fimmu.2016.00072. eCollection 2016. Front Immunol. 2016. PMID: 26973649 Free PMC article.
-
Both viral and host factors contribute to neurovirulence of bovine herpesviruses 1 and 5 in interferon receptor-deficient mice.J Virol. 2004 Apr;78(7):3644-53. doi: 10.1128/jvi.78.7.3644-3653.2004. J Virol. 2004. PMID: 15016885 Free PMC article.
-
Redox Control of the Immune Response in the Hepatic Progenitor Cell Niche.Front Cell Dev Biol. 2020 May 6;8:295. doi: 10.3389/fcell.2020.00295. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32435643 Free PMC article.
-
Administration of probiotics influences F4 (K88)-positive enterotoxigenic Escherichia coli attachment and intestinal cytokine expression in weaned pigs.Vet Res. 2011 May 23;42(1):69. doi: 10.1186/1297-9716-42-69. Vet Res. 2011. PMID: 21605377 Free PMC article.
-
NK cells and gamma interferon coordinate the formation and function of hepatic granulomas in mice infected with the Francisella tularensis live vaccine strain.Infect Immun. 2008 Apr;76(4):1379-89. doi: 10.1128/IAI.00745-07. Epub 2008 Jan 28. Infect Immun. 2008. PMID: 18227174 Free PMC article.
References
-
- Allen, R. G., and M. Tresini. 2000. Oxidative stress and gene regulation. Free Radic. Biol. Med. 28:463-499. - PubMed
-
- Baron, J. L., L. Gardiner, S. Nishimura, K. Shinkai, R. Locksley, and D. Ganem. 2002. Activation of a nonclassical NKT cell subset in a transgenic mouse model of hepatitis B virus infection. Immunity 16:583-594. - PubMed
-
- Behar, S. M., and S. Cardell. 2000. Diverse CD1d-restricted T cells: diverse phenotypes, and diverse functions. Immunology 12:551-560. - PubMed
-
- Bendelac, A., M. N. Rivera, S.-H. Park, and J. H. Roark. 1997. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu. Rev. Immunol. 15:535-562. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

