Cleavage of the human immunoglobulin A1 (IgA1) hinge region by IgA1 proteases requires structures in the Fc region of IgA
- PMID: 12704129
- PMCID: PMC153282
- DOI: 10.1128/IAI.71.5.2563-2570.2003
Cleavage of the human immunoglobulin A1 (IgA1) hinge region by IgA1 proteases requires structures in the Fc region of IgA
Abstract
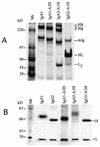
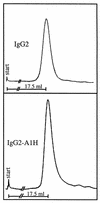
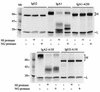
Secretory immunoglobulin A (IgA) protects the mucosal surfaces against inhaled and ingested pathogens. Many pathogenic bacteria produce IgA1 proteases that cleave in the hinge of IgA1, thus separating the Fab region from the Fc region and making IgA ineffective. Here, we show that Haemophilus influenzae type 1 and Neisseria gonorrhoeae type 2 IgA1 proteases cleave the IgA1 hinge in the context of the constant region of IgA1 or IgA2m(1) but not in the context of IgG2. Both C(alpha)2 and C(alpha)3 but not C(alpha)1 are required for the cleavage of the IgA1 hinge by H. influenzae and N. gonorrhoeae proteases. While there was no difference in the cleavage kinetics between wild-type IgA1 and IgA1 containing only the first GalNAc residue of the O-linked glycans, the absence of N-linked glycans in the Fc increased the ability of the N. gonorrhoeae protease to cleave the IgA1 hinge. Taken together, these results suggest that, in addition to the IgA1 hinge, structures in the Fc region of IgA are required for the recognition and cleavage of IgA1 by the H. influenzae and N. gonorrhoeae proteases.
Figures

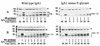

References
-
- Baenziger, J., and S. Kornfeld. 1974. Structure of the carbohydrate units of IgA1 immunoglobulin. I. Composition, glycopeptide isolation, and structure of the asparagine-linked oligosaccharide units. J. Biol. Chem. 249:7260-7269. - PubMed
-
- Baenziger, J., and S. Kornfeld. 1974. Structure of the carbohydrate units of IgA1 immunoglobulin. II. Structure of the O-glycosidically linked oligosaccharide units. J. Biol. Chem. 249:7270-7281. - PubMed
-
- Chintalacharuvu, K. R., M. Raines, and S. L. Morrison. 1994. Divergence of human alpha-chain constant region gene sequences. A novel recombinant alpha 2 gene. J. Immunol. 152:5299-5304. - PubMed
-
- Chintalacharuvu, K. R., L. J. Yu, N. Bhola, K. Kobayashi, C. Z. Fernandez, and S. L. Morrison. 2002. Cysteine residues required for the attachment of the light chain in human IgA2. J. Immunol. 169:5072-5077. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

